A sample of gold weighing 28.4 g was heated to 500. ÐC and
plunged into 100. grams of water at 25.34 ÐC. Calcualte the
final temperature of the mixture.
Answer
We'll solve this problem just as we did the problem using two
samples of water at different temperature,
heatgold = –heatwater
In this case heat of the gold is transferred to the water. To
solve the problem we must substitute for heat using the
relationship between heat and specific heat, so substituting
yields,
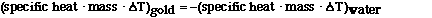
Now we substitute in the quanities given in the problem and
solve. Substituting we have,
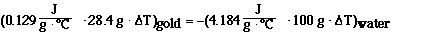
Simplfying,
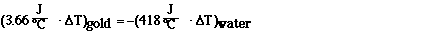
and

Now we replace the temperature information,

The initial temperature, (Ti)gold, for
the gold is 500 °C, and the initial temperature, (Ti)water,
for the cool water is 25.34 °C. The final temperature for the
gold and the cool water is the same because we are mixing the two
samples together. So substituting,
(Tf - 500 °C) = –114(Tf
- 25.34 °C)
(Tf - 500 °C) = –114Tf
+ 2889 °C
Collecting terms on opposite sides,
115Tf = 3389 °C
Tf = 29.47 °C
![]()
![]()
![]()