
 |
Obtain a 24-well plate and a conductivity apparatus from the storeroom. |
 |
1. Obtain a small sample of each of the solids listed in the first RESULTS box and place them in a row on a clean paper towel. |
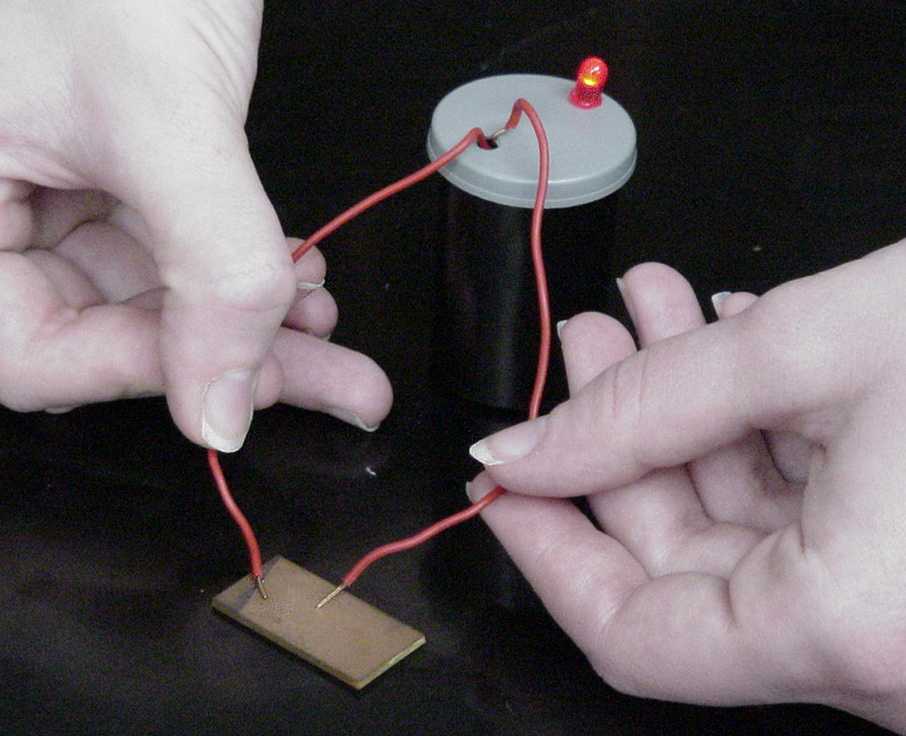 |
 |
 |
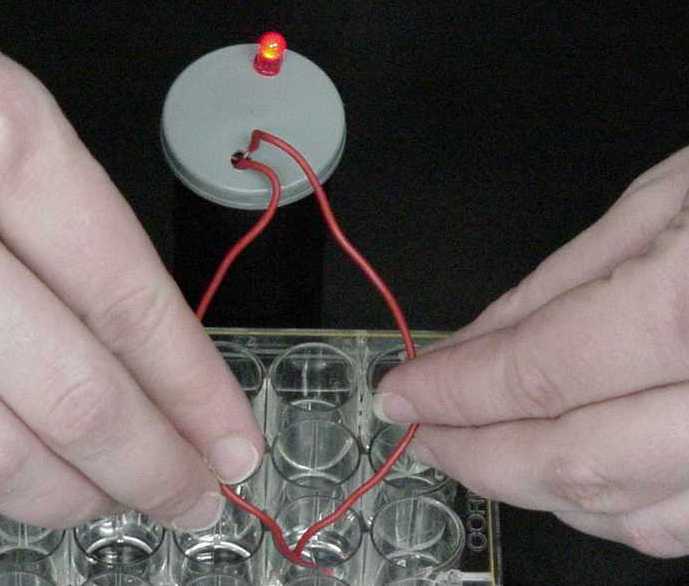 |
 |
Here, the LED glows brightly. The solution is conductive and a strong electrolyte. |
 |
This sample causes the LED to glow faintly. This solution is semi-conductive and a weak electrolyte. |
 |
When testing this solution, the LED does not glow. This sample is not a conductor and is a non-electrolyte. |