
 |
Locate the sand, silt, and salt reagent bottles. |
 |
2. Place an appropriate amount of each solid into its test tube. ( Each reagent bottle has a test tube attached to its side that shows you the approximate amount that is needed). |
 |
3. To each solid, add a few millimeters (about one inch) of distilled water. Gently flick side of each test tube with your fingers to thoroughly mix the contents. |
 |
4. Record your solubility observations in the first RESULTS box. |
 |
2. Weigh a piece of filter paper, a watch glass, an evaporating dish, and ( as shown) a 50ml beaker. Record the masses in the third RESULTS box. |
To weigh out a sample of the unknown mixture we need to first tare a beaker and a test tube. Taring the beaker and the test tube will allow us to eliminate their masses from any calculations. Taring is the same as 'zeroing' the mass of these items. (NOTE: To replay the video click on the small arrow on lower left portion of the image.) |
|
Having tared the beaker and test tube we are now ready to measure out approximately 1 gram of the unknown mixture. Notice this particular student has measure more than 1 gram. This is no biggy, we only need approximately 1 gram. If it is a little more, as in this case, or a little less we can still complete the experiment. (NOTE: To replay the video click on the small arrow on lower left portion of the image.) |
 |
5. Add a few milliliters (about one inch) of distilled water to the unknown. |
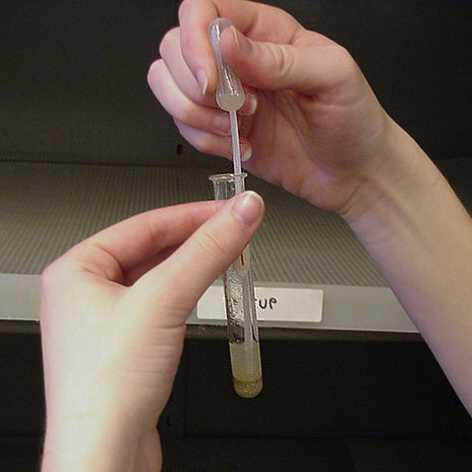 |
6. Use the disposable pipet to carefully draw off the liquid suspension, leaving the solid portion behind. Squirt the contents of the pipet into test tube #2. |
 |
8. Set up a filtration apparatus as shown. (Go here to see how to fold the filter paper shown in the filter funnel. Go here to see how to place and hold the filter paper in the filter funnel.) Carefully pour the liquid suspension in test tube #2 onto the filter paper in the funnel, allowing the filtrate to collect into the 50ml beaker. Rinse the test tube with as little distilled water as possible ( the more you use, the longer it will take to dry). Pour the rinsing onto the filter paper as well. |
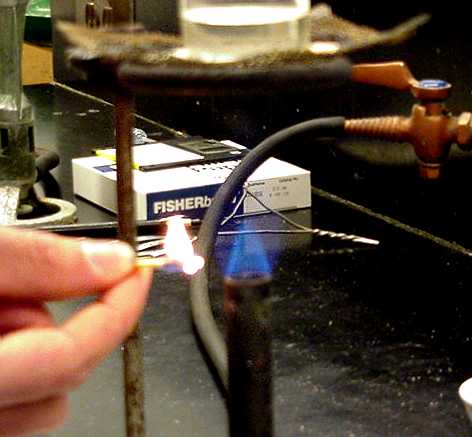 |
Use caution when lighting a Bunsen burner. Always remember to light the match first, then turn on the gas. Light the flame by approaching the side of the burner, as shown here. |
 |
Heat the beaker very gently to avoid spattering . This will evaporate the water from the solution. |
 |
10. Remove the filter paper from the funnel and place it onto the pre-weighed watch glass. Set the watch glass under a heat lamp and allow it to dry. |
 |
12. Set the evaporating dish onto another wire gauze supported by a ring and ring stand and using a second Bunsen burner, evaporate the water from the dish. |
 |
13. When the 50mL beaker, the watch glass, and the evaporating dish are completely dry, allow them to cool. |