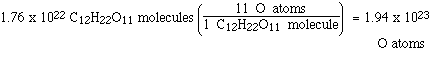Consider the balanced equation

We can balance the equation and we recognize it is an example
of a formation reaction. How do we read this equation?
This equation reads 1 atom of magnesium combines with one
molecule of chlorine to form one formula unit of the ionic
compound magnesium chloride. If we wanted to do this reaction to
prepare some magnesium chloride it would be difficult to find a
single atom of magnesium and a single molecule of chlorine, even
for the most experienced chemist. In the chemistry laboratory we
must measure amounts of substances by mass. What is the
relationship between the amount of substance and the number of
atoms or molecules? How are we able to perform quantitative
chemical reactions? There is an answer!
Lets return to the periodic table. In the periodic table the
mass number for an element is given below the symbol.
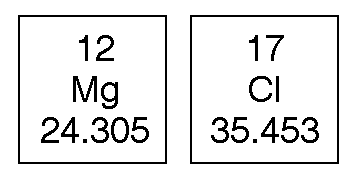
The relative weighted average atomic mass for
magnesium is 24.3 u. This is obtained by summing the product of
the mass of each isotope of the element times the fractional
abundance of that isotope. We usually shorten this and say the
atomic mass of magnesium is 24.3 u. The atomic mass for chlorine
is 35.45 u. The symbol for chlorine is Cl. The formula for
elemental chlorine is Cl2. The molecular mass for
elemental chlorine, Cl2, is 70.90 u, just two times
the atomic mass. We can obtain the mass of a formula unit of MgCl2
by simply summing the atomic masses for each of the atoms in the
formula. Therefore the formula mass of MgCl2 is 95.35
u.
But we still have a problem! The balances in the
laboratory measure mass in units of grams, not u's. We need a
relationship between atomic mass units (u) and grams. That
relationship is given to us by the definition of the atomic mass
unit. The 'u' is defined as one-twelfth of the mass of the
isotope of 12C. The value is 1.66057 x 10-24
grams. Now we have a way to determine the mass of a single atom
of an element, a molecule of an element or compound and the mass
of a formula unit of an ionic compound.
Let's see how we can use this information to
calculate the mass of an atom of magnesium in grams. First we can
obtain the atomic mass of a magneium atom. From the periodic
table this value is 24.305 u. Now we can use a unit factor to
convert from u's to grams.
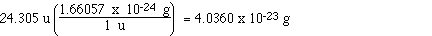
This is a very small number!
Calculate the mass of a molecule of chlorine in
grams; The formula of chlorine is Cl2 so its molecular
mass is 2 x 35.453 u which is 70.906 u. Now we can use a unit
factor to convert from u's to grams;
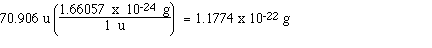
Finally, let's go ahead and calculate the mass
of a formula unit of magnesium chloride in grams; The formula of
magnesium chloride is MgCl2 so its formula mass is
24.305 u + 2 x 35.453 u which is 95.211 u. Now we can use a unit
factor to convert from u's to grams;
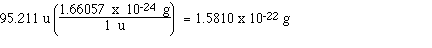
So let's try one more substance. Calculate the
mass, in grams, of a molecule of sucrose, C12H22O11.
First the molecular mass of the compound
must be determined;
for C: 12 x 12.0 u = 144 u
for H: 22 x 1.01 u = 22.22 u
for O: 11 x 16.0 u = 176 u
Total = 342 u (molecular mass for one
molecule of C12H22O11)
Now convert from units of 'u' to units of
grams;
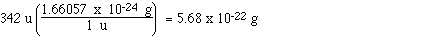
In all of the above examples we have determined
the mass of a single atom, molecule or formula unit of a
substance. Let's try a different kind of calculation which
extends the ideas a little further.
Calculate the number of magnesium atoms in 10.0
g of magnesium.
In this question we need to determine how
many magnesium atoms are contained in the 10.0 gram sample.
If we knew the mass of one magnesium atom we could use that
information to determine how many magnesium atoms were in
10.0 grams. We do know how much one magnesium atoms weighs,
we calculated it above. We know the atomic mass of magnesium
is 24.305 u. When we convert that to grams that is the mass
of one magnesium atom. We used the conversion that 1 u =
1.66057 x 10-24 grams, as shown below.
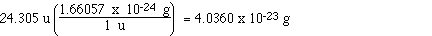
So the mass of one magnesium atom is 4.0360
x 10-23 grams. Now we can determine the number of
atoms in 10.0 g of magnesium,
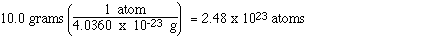
As one additional calculation using this same
approach let's calcualte the number of magnesium atoms in 24.305
g. Since we already know the mass of one magnesium atom we can
set this problem up just as we did above.
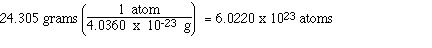
We see that in 24.305 g of magnesium there are
6.022 x 1023 atoms of magnesium.
Now calculate how many sucrose molecules there
are in 10.0 grams of sucrose.
To answer this question we need to know the
mass of a single sucrose molecule. We calculated that earlier
and it was 5.68 x 10-22 grams. Therefore, the
number of molecules of sucrose in 10.0 grams is;
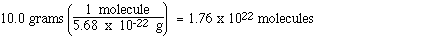
How many oxygen atoms are there in 10.0 grams of
sucrose?
We know how many sucrose molecules there are
in 10.0 grams and we also know how many oxygen atoms there
are in each sucrose molecule. We can answer the question with
those two pieces of information;
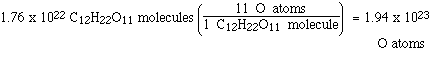
Now let's consider a similar question asked
above, how many molecules of sucrose are there in 342 grams of
sucrose?
We solve this question just like the
question about the number of molecules in 10.0 grams,
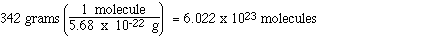
We see that in 342 g of sucrose there are 6.022
x 1023 molecules of sucrose.
Earlier we also determined there are 6.022 x 1023
atoms of magnesium in 24.305 g of magnesium.
Do you notice something interesting?
![]()
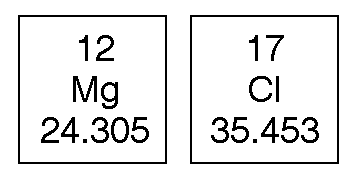
![]()
![]()
![]()