The interesting observation is that both 24.305 grams of
magnesium and 342 grams of sucrose have the same number of
particles. The number of magnesium atoms in 24.305 grams of
magnesium is the same as the number of sucrose molecules in 342
grams of sucrose. That number is 6.022 x 1023.
This number is special, it is called Avogadro's number and it
is a fundamental unit in the SI system. Specifically this number
is called a mol and is defined as the number of 12C
atoms in 12 g of 12C. This number has been
experimentally determined as 6.022 x 1023 atoms. A mol
of any substance is defined as the amount of the substance which
contains the same number of units as are in 12 g of 12C
or 6.023 x 1023 units of the substance.
So a mol is several things; it is an amount of substance
which contains the same number of atoms, molecules or formula
units as 12 grams of 12C. We saw earlier than 24.305
grams of magnesium and 342 grams of sucrose contain the same
number particles. In the case of magnesium it is atoms, in the
case of sucrose it is molecules. But whichever...atoms or
molecules...the number is the same. Therefore we refer to 24.305
grams of magnesium as one mol of magnesium. Similarly 342 grams
of sucrose is one mol.
How many grams of chlorine, Cl2, are in one mol?
This can be determined by finding the molecular mass of
Cl2.
2 x 35.453 u = 70.906 u
The molecular mass of Cl2 is 70.906 u. When
this mass is expressed in units of grams, 70.906 grams, that
mass of chlorine contains 6.022 x 1023 molecules
of Cl2 and is also a mol. So there are 70.9 grams
in one mol of Cl2.
A mol of 12C atoms contains 6.023 x 1023
atoms of 12C.
A mol of Cl2 molecules contains 6.023 x 1023
molecules of Cl2.
A mol of MgCl2 contains 6.023 x 1023
formula units of MgCl2.
A mol of 12C atoms weighs the same in grams as the
atomic mass of 12C, 12 g. The weight of any substance
which contains 6.023 x 1023 units is given by its
atomic, molecular or formula mass expressed in grams and is
called the molar mass of the substance.
A mol of 12C atoms has a mass 12 g: the molar mass
of 12C is 12 g.
A mol of Cl2 molecules has a mass 70.90 g: the
molar mass of Cl2 is 70.90 g.
A mol of MgCl2 has a mass 58.5 g: the molar mass
of MgCl2 is 95.2 g.
Sample Problem:
Determine the number of moles of N2O5
in 45.6 g of N2O5 gas and the number of
nitrogen atoms.
Solution:
The molar mass of N2O5 is 108
gmol-1. The sample is 45.6 g so we know there is
less than a mol since a mol of N2O5 weighs
108 g. To calculate the number of moles we setup the problem
in the following way;
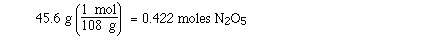
To determine the number of nitrogen atoms in
the sample we must first determine how many N2O5
molecules.

Now we can determine the number of nitrogen
atoms;

![]()
![]()