|
To begin we looked at the conductivity of a
sample of deionized water (water with little or no ions)
which could be viewed as pure water. Looking at the image
on the left the light bulb is not glowing. This can be
explained in terms of the nature of the species in the
sample of water. That the light does not glow suggests
there are no ions in the sample. This is an important
measurement to establish subsequent measurements in
deionized water. Any observed conductivity will due to
the presence of the solute.
|
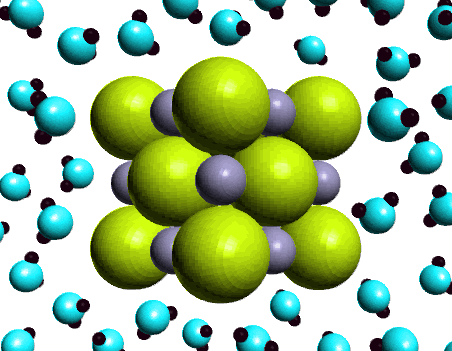
Next we measured the conductivity of an aqueous
solution of sodium chloride. Here the light bulb glows
brightly indicating the presence of ions in the solution.
The ability of an aqueous solution of sodium chloride to
conduct electricity is characteristic of a strong
electrolyte. How do we express this observation using a
chemical equation?
NaCl(s) ---H2O-->
Na+(aq) + Cl-(aq)
Soluble ionic compounds are also strong electrolytes,
i.e., behave the same way as sodium chloride...forming
ions when dissolved in water. How do we know whether an
ionic compound dissolves in water? That is determined by
experiment. A Solubility Table summarizes the
experimental observations of the solubility behavior of a
large group of ionic compounds. We'll discuss a
Solubility Table shortly.
|
.gif)
|

|
When we tested the conductivity of an aqueous
solution of sucrose the light bulb did not glow. This
type of behavior is characteristic of a nonelectrolyte.
No ions in this solution, yet the sucrose dissolved. How
do we write a chemical equation in this case?
C12H22O11(s)
---H2O--> C12H22O11(aq)
In general soluble covalent compounds do not
dissociate into ions when dissolved in water.
|