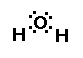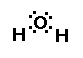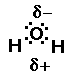Water is the most abundant, most accessible and one of the
most studied chemical compounds. The fact that it is located
throughout the planet, its crucial importance to man's survival
and its ability to exist in solid, liquid and vapor phases and
transform between them readily has made this liquid uppermost in
the thinking of man throughout time.
Water plays an important role in many religions and in 500 BC
was considered by many secular philosophers as the sole
fundamental principle in nature. Thales of Miletus said,"It
is water that, in taking different forms, constitutes the earth,
atmosphere, sky, mountains, gods and men, beasts and birds, grass
and trees, and animals down to worms, flies and ants. All these
are different forms of water. Meditate on water." Although
this sounds strange and perhaps farfetched, even to the ears of
freshman chemists, we should remember that many marine
invertebrates are 96 - 97 % water and the human embryo, during
its first month, is 93 % water. (As Steve Wright, the deadpan
comedian, would say we are this far {show two finger barely
separated from each other} from drowning.) Aristotle consider
water as one of the four elements along with, earth, fire and
air.
Water is distributed very unevenly around the globe with
97.33 % as salt water and 2.67 % as freshwater. One can
understand with so little fresh water why purification and
recycling is so important. Lakes and
streams account for only 0.01% of all the water on our planet.
Groundwater is the largest accessible reserve but still accounts
for only 0.6% of the total water and only about half of this is
within reach of even the deepest wells. Water makes up
about 0.023 % of the total mass of the earth, with about 1.38 x
1021 kg distributed, on, and below the earth's
surface. The volume of this amount of water is 1.4 billion cubic
kilometers. Over 80 % of the surface of the earth is covered by
water (ice caps, lakes and streams, and oceans). Water is
essential to most forms of life and accounts for more than 60 %
of the mass of the human body.
There are 328 million cubic miles, or
3.612 x 1020 gallons
(361,200,000,000,000,000,000 gallons) of sea water in the ocean.
If all that water were piled on top
of the United States the land would be submerged under 88.2 miles
of water. If the same amount of sea water was to be piled up onto
all of the available land above sea level on this planet, the
land would be 5.7 miles below the water's surface.
Water is really pretty interesting.
Most solids expand up to 10 % of their volume when they melt.
Water expands by the same amount when it freezes!
Most solids are more dense than the liquid phase. However,
ice has a density of 0.917 g/cm3 while liquid water has a density
of 0.998 g/cm3. Lakes freeze from the top down, insulating the
liquid water below. If the density of the solid were greater than
that of water, lakes would freeze from the bottom up and all the
animal and plant life in the aquatic environment would die.
Water has a melting point which is 100 degrees C higher than
expected for its group of hydides.
Water has a boiling point 200 degrees C higher than expected
for its group of hydides.
Water is a liquid at room temperature. The hydrogen compounds
around oxygen are all toxic gases, CH4, NH3,
H2S, HF.
Water has the highest surface tension of any liquid except
mercury. High surface tension helps plants move water from the
earth surface up into the tree limbs and leaves.
Water is an excellent solvent, dissolving many ionic
compounds which are insoluble in other compounds.
Water has the highest heat of vaporization of all known
substances. The heat needed to vaporize 1 g of water at 100
degrees C is 20,000 joules. The result is perspiration when it
evaporates from our body cools because the heat from our body is
absorbed by the water as it changes phase from liquid to vapor.
Water has a high heat capacity. That means it can absorb
large amounts of heat without large changes of temperature. Its
heat capacity is 10 times copper or iron. This property accounts
for the moderating influences on the climate around cities near
large bodies of water. Without water the temperature changes
between day and night would be much greater than we experience.
Large bodies of water release heat absorbed from the sun during
the day, at night, or during the winter. Most
of the water is contained in the
oceans and the high heat capacity of this large volume of water
(1.35 million cubic kilometers) buffers the Earth surface from
large temperature changes such as
those observed on the moon.
All of these seemingly anomolous properties can be understood
in terms of the intermolecular attractive forces which exist
between water molecules. The particular intermolecular attractive
force is called hydrogen-bonding.
Lets look at a simulation which gives us an idea of what is
meant by the term attractive
forces.
What follows is a narrative of what I might say during the
attractive forces animation. You might print these notes out so
you could read along as the simulation ran.
Let's begin by considering a sample of a gas. We will view
this sample of gas in terms of the kinetic-molecular model. That
is a gas consists of a collection of widely spaced particles in
constant, chaotic motion. The kinetic energy of the gas
particles, which is proportional to the temperature of the gas,
is much greater than any attractive force that exists between the
particles. So all collisions occuring between particles are
elastic.
As we cool a gas, lower its temperature, the molecule's
velocity drop and they will begin to 'stick' together. This is
not a chemical bond being formed but an attraction that molecules
feel for each other which is not observed at high temperature
because of the high kinetic energy of the molecules. In a gas
when the particles are widely spaced there are no attractive
forces between the particles. However, as the temperature drops
the velocity of the particles drops and they come closer
together.
The intermolecular attractive forces in a liquid are
sufficient to hold the particles in close proximity, yet there is
still chaotic motion. So liquids can flow. In liquids the
particles are closer together, and the density of liquids are
greater than gases. There is very little free space between
particles in the liquid phase, so liquids are not very
compressible. Liquids have definite volumes, but do take the
shape of the container which they occupy.
In solids the intermolecular forces are sufficiently strong
relative to the kinetic energies that molecules are virtually
locked in place. Each particle occupies a certain position
relative to its neighbors often in a regular pattern that extends
throughout the solid. Solids, like liquids are not very
compressible because of the lack of space. Because particles are
not free to undergo long range movement solids are rigid they do
not flow. Translational motion is restricted, but they can
vibrate in position.
The 'stickyness' exhibited by particles at lower temperature,
which result in the formation of liquids and eventually solids is
due to intermolecular attractive forces. Intermolecular means
between molecules. Intramolecular means between atoms.
Intramolecular forces are what we call covalent bonds and are
very strong (100 - 1000 kJ/mol). Intermolecular forces are
between molecules and are weak (.1 - 40 kJ/mol). It is
intermolecular forces which explain the formation of liquids and
solids in covalent compounds. The intermolecular attractive
forces are electrostatic in nature.
Water is an interesting compound! Lets consider its
structure..its geometry. What is the shape of water? We can
answer this question by drawing its Lewis structure. Water has a
central oxygen atom with two lone-pairs of electrons and two
bonding pairs of electrons. The geometry about water is bent.
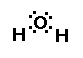
The oxygen has two unpaired electrons occuping orbitals
around the oxygen. Those electrons are not balanced by the
electrons bonding to the hydrogen atoms. Oxygen is sharing two
pairs of electrons with the hydrogen. But because oxygen has so
many more protons than hydrogen it tends to hold the electrons
closer to it than do the hydrogens. The result is oxygen has some
partial negative charge on it, causing the hydrogens to have a
partial positive charge. This makes water like a small magnet.
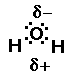
Water is called a polar compound. Anytime a central atom has
a lone pair of electrons the compound is most likely polar. Polar
compounds act like little magnets and are attracted to each
other. The unique properties of water are a result of the shape
of the water molecule and its polarity.
The boiling points of the Noble Gas elements and the hydrides
of Groups IV - VI show some interesting patterns. Here is an interactive
movie of these boiling points. Notice the boiling point of
the Noble Gases increases with increasing molar mass. This is as
expected. Proceeding down the group each succeeding Noble Gas has
more electrons. It is larger, more massive and more polarizable.
Since the Noble Gases are monoatomic the only intermolecular
attractive force which occurs between adjacent atoms are of
London Dispersion type. The more electrons, the more polarizable,
the stronger the instantaneous-induced dipole interaction the
higher the boiling point. The trend for the nonpolar Group IV
hydrides is similar, as is the explanation of the observed trend.
However, in the Group V, VI and VII hydrides an unusual
observation is made. The lightest member of each series has
boiling point which is significantly higher than would be
predicted. (Note: Predicting the boiling point of the lightest
member would be accomplished by extrapolation from the data of
three heavier members of a series.) In the case of water it is
200 degree higher than expected. Clearly the intermolecular
attractive forces occurring in H2O, NH3 and HF are significantly
stronger compared to the other members of a series.
A site at New York University has an introduction to
hydrogen-bonding interactions in water and ice. This site also
has several very nice animations depicting the hydrogen-bonding
interaction between water
molecules. Another page at this site provides VRML
and PDB views of different numbers of water molecules. It
should be noted that Mac users may have trouble viewing the VRML
versions of the water structures at this site.
Here is an mpeg movie which shows two
water molecules interacting in the vapor phase at 400 K. This
movie was produced by Dr. Lars Ojamäe is an assistant professor
at the Department of Physical Chemistry, Stockholm University,
Stockholm, Sweden.
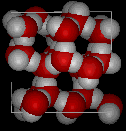
Here are images of water in the liquid phase
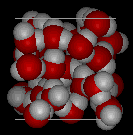
and water in the solid phase.