So now we need to talk about the other number, the one below
the symbol. This number is the relative, weighted average atomic
mass of all of the isotopes of the element. So how did it come to
that?
It started with early chemists and their explorations to try
to understand the nature of chemical substances. Everyone about
water, H2O...whoops I can not call it H2O
because in the early days know one knew the formula for water.
They did know it contained the elements hydrogen and oxygen
because every sample of water was found to contain 88.81% oxygen
and 11.19% hydrogen. That was the composition of water. So in a
100 g sample of water there is always 88.81 g of oxygen and 11.19
grams of hydrogen. In terms of a mass ratio if we divideboth the
numbers by the smallest number we get;
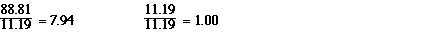
In terms of mass ratios water contains about 8 times more
oxygen than hydrogen. However much hydrogen is in water, there is
8 times more oxygen. Now atoms were a big deal than and the
scientific community agreed that matter was composed of atoms. So
water contained hydrogen and oxygen atoms. And everyone agreed
that oxygen weighed more than hydrogen. But no one knew how much
a hydrogen atom weighed or how much an oxygen atom weighed. So it
required guessing the formula for water and than supporting the
guess on the basis of experimental data. In fact the first guess
for the formula for water was OH, hey why not..keep it simple!
Right? On further investigation and additional experimental
evidence Avogadro proved the formula for water had to be H2O.
Sceintists were able to arrive at the correct formula for water
without even knowing the actual mass of a hydrogen or oxygen
atom.
Well now we do know the mass of atoms. We use an instument
called a mass spectrometer to determine that kind of information.
The mass of a hydrogen atom has been found to be 1.6732 x 10-27
kg. The mass of an oxygen atom is 2.6555 x 10-26 kg.
So we see that there is 7.94 times as much oxygen in water as
there is hydrogen. The same number that scientists found many,
many years ago.
But the numbers in the periodic table for the atomic mass of
hydrogen do not look like the masses I've given above...what
gives? This is because the masses in the periodic table are
RELATIVE. The mass for hydrogen and for oxygen given above are
the actual masses. So what are the numbers in the periodic table
relative too? They are relative to the isotope of carbon-12, 12C.
So how is it made relative?
The actual mass of an atom of 12C is 1.99268 x 10-26
kg. Now the next thing is to take 1/12 (one-twelfth) of that
mass. When we do that we get;
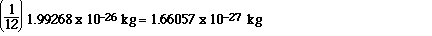
OK, now the definition...1 atomic mass unit is defined as
1.66057 x 10-27 kg. So to get the relative atomic mass
of a hydrogen atom in atomic mass units we simply divide.

Now, this number is real close to the number in the periodic
table. It is not exactly the same because hydrogen is made up of
three isotopes. When the mass of the other two isotopes is
included the relative, weighted average atomic mass of hydrogen
is 1.00794 u.
So why do the relative thing? First writing the actual mass
of the elements takes too much space. Probably even more
important is historically the combining mass of hydrogen was
equal to 1.00 g, and for oxygen it was 16.0 g. So by doing
relative atomic masses as I've described above we get values like
the historically determined combining masses.
![]()
![]()
![]()