 |
The green (Bat Man) balloon contains carbon
dioxide gas. (The woman in the video is Nancy Gettys who
worked with me in the early 90's teaching a chemistry
course.)
|
 |
Here Nancy is placing the balloon in an
evaporating dish with some liquid nitrogen which she has
poured from the dewar near her left hand.
|
 |
As you can see the volume of the balloon
containing the carbon dioxide is getting smaller.
|
 |
Here the volume of the carbon dioxide gas in the
balloon is effectively zero. All of the carbon dioxide
gas has formed a solid, so now the balloon contains only
solid carbon dioxide.
|
 |
Now the balloon has been removed from the liquid
nitrogen and the carbon dioxide in the balloon is warming
up.
|
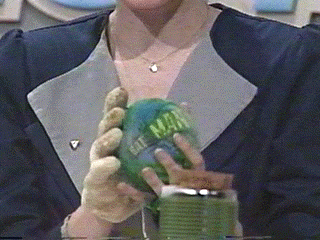 |
Here the balloon and the carbon dioxide is warmed
even more. Notice the volume of the balloon is
increasing. As the carbon dioxide solid warms it
sublimes, that is, it changes from the solid state to the
gaseous state directly. It turns out the liquid state for
carbon dioxide does not exist under normal atmospheric
presure conditions.
|
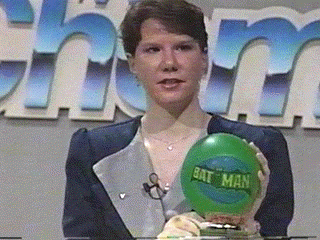 |
Finally the balloon has returned to its initial
volume, all of the carbon dioxide has returned to the gas
phase.
|