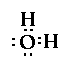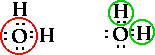So draw the structure of each of the following
compounds, determine the number of bonding pairs and
nonbonding pairs of electrons on the central atom and
identify the geometry/shape of the molecule.
a) CCl4
This compound contains a carbon atom and four
chlorine atoms. Drawing the electron structure of the
individual atoms,
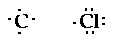
The carbon needs four electrons to have eight and
each chlorine requires one electron to have eight. So the
structure (four chlorines around the carbon) would look
like,
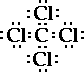
The central carbon atom has 4 bonding pairs of
electrons and no nonbonding pairs. So the geometry/shape
of the molecule is tetrahedral.
b) NF3
This compound contains a nitrogen atom and three
fluorine atoms. Drawing the electron structure of the
individual atoms,
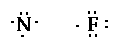
The nitrogen needs three electrons to have eight and
each fluorine requires one electron to have eight. So the
structure (four fluorine around the nitrogen) would look
like,
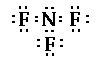
The central nitrogen atom has 3 bonding pairs of
electrons and one nonbonding pairs. So the geometry/shape
of the molecule is pryramidal.
c) H2CO
This compound contains a carbon atom, an oxygen atom
and two hydrogen atoms. Drawing the electron structure of
the individual atoms,
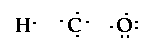
Now this is starting to look a little
complicated. But not really. The hydrogens in the formula
are next to the carbon so we'll have them bond to the
carbon, and well have the oxygen bond to the carbon also.
So the structure would look like,
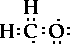
But this looks funky. Carbon does not have eight
electrons, but seven and the oxygen also has seven
electrons. But both need eight! The hydrogen atoms are
happy. But how do we handle the carbon and the oxygen.
Since they each have an unpaired electron, why don't we
pair those electrons. But where do we put the pair? Well
we'll put it in the same place as the other pair. Since
one of the electrons is carbon's and one is oxygens and
they both need one more electron...why not!?
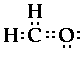
The central carbon atom has eight electrons around it
as does the oxygen atom.
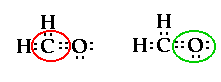
The two pairs of electrons between the carbon and
oxygen atom are called a double bond. So the central
carbon atom still has four pairs of electrons around it.
But not like the four pairs of electrons in CH4.
In this case it has two bonding pairs and one double
bonding pair and no nonbonding pairs. The geometry for
this shape is called trigonal planar.
d) C2H6
This compound contains two carbon atoms, and six
hydrogen atoms. Drawing the electron structure of the
individual atoms,
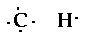
This is looking complicated also. Lts
try to reason this one through. We know carbon likes to
form four bonds. So how do we distribute the hydrogens,
and what do the carbons do. The fact that the formula is
C2H6 means the molecule contains
two carbon atoms and six hydrogen atoms bonded together
in some way. If we try to put four of the hydrogens
around one of the carbons that would use up all the bonds
to that carbon and we would still have a carbon atom and
two hydrogen atoms left over wondering what should be
done with them?! It turns out that carbon like to bond to
itself and since we have two carbon atoms lets bond them
together,
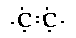
Notice there are six unpaired electrons
around the two carbon atoms, and we have six hydrogen
atoms that we need to include. So it seems obvious to
bond each electron ina hydrogen atom with the unpaired
electron on the carbon atoms.
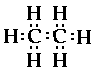
Each carbon atom has four bonding pairs of electrons
around it so each carbon atom has a geometry/shape of
tetrahedral. What does that look
like!?
|
![]()