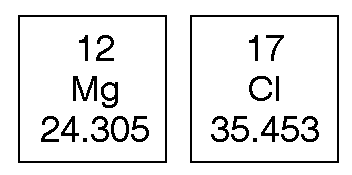Problem 1:
How many atoms in 24.022 u of carbon?
Since 1 carbon atom weighs 12.011 u we can do the following calculation,
![]()
Problem 1:How many atoms in 24.022 u of carbon?Since 1 carbon atom weighs 12.011 u we can do the following calculation,
|
Problem 2:How many atoms in 5.583 x 10-22 g of carbon?Since 1 carbon atom weighs 1.994 x 10-23 g we can do the following calculation,
|
a) 526.56 u of phosphorus atoms
b) 6.918 x 10-23 g of lithium atoms (note the mass of one lithium atom is 1.153 x 10-23 g)
Problem 3:Calculate the number of carbon atoms in 12.0011 g of carbon.
So 12.011 g of carbon contain 6.023 x 1023 C atoms.Problem 4: How many carbon atoms in 28.0 g of carbon?If there are 6.023 x 1023 C atoms in 12.011 g of carbon, then
|
a) 55.85 g of iron atoms
b) 28.085 g of silicon atoms
c) 56.17 g of silicon atoms
Problem 5:Calculate how many moles in 12.011 g of carbon.Since 1 mol of carbon is equal to its relative, weight average atomic mass expressed in grams. than in this case there is 1 mol in 12.011 g of carbon.Problem 6:Calculate how many moles in 35.8 g of carbon.Here we solve the problem similar to others we have been doing. Since 1 mol of carbon weighs 12.011 g I just need to divide.
Problem 7:Calculate how many moles in 2.12 x 1023 atoms of Zn. 1 mol of Zn has 6.023 x 1023 atoms therefore we need to set this problem up in the following way,
|