Answer
Draw the structure of ammonia and determine the number of
bonding pairs of electrons and the number of lone-pair electrons.
Ball-and-Stick
|
Lewis Structure
|
Electron-dot Structure
|
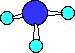
|
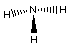
|
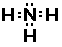
|
Notice there are three bonds to the nitrogen in this compound
and a lone-pair of electrons on the nitrogen. We can only see the
lone-pair in the right most Electron-dot structure. It is present
in the other two structures, it just is not shown.
Amines can be thought of as derivatives of ammonia. Amines
contain at least one carbon containing substituent bonded to the
nitrogen in place of one of the hydrogen atoms. So if we replace
one hydrogen atom with the simplest carbon containing branching
group we would have the following compound, CH3NH2.
Draw the structure of this compound?