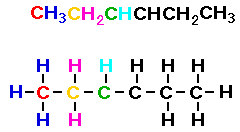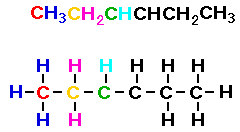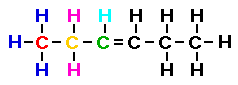Reading condensed formula of organic compounds
The most important thing to remember is the number of bonds
carbon forms. How many bonds does carbon want to form in the
organic compounds we have been studying?
Answer: 4 bonds
This is very important along with the fact that hydrogen only
needs one bond. So lets look at a condensed formula and see how
we would 'see' it.
Consider CH3CH2CHCHCH2CH3.
Is this an alkane, an alkene or an alkyne?
Answer: We need to write the formula in
the form CwhateverHwhatever. In this case
writing the formula we get C6H12. This is
the formula of an alkene. Remember alkenes have the general
formula CnH2n.
So since this compound is an alkene we know it contains a
double bond. Where is it located in this condensed formula? To
answer this question we need draw a Lewis structure, either on
paper or mentally and associate the hydrogens with the correct
carbons as described in the formula. An important thing to
remember is the the hydrogen(s) associated with a particular
carbon are generally written to the right of the carbon atom.
(Although sometimes the hydrogens are written to the left, but
usually only for the left-most carbon in the formula.)
Using this information draw the Lewis structure showing the
carbons and the hydrogens?
Answer.
In the condensed formula shown below I have colored the first
three carbon atoms and the hydrogen atoms associated with those
carbon atoms. Then I drew the lewis structure showing the
relationship between the condensed formula and the Lewis
structure.
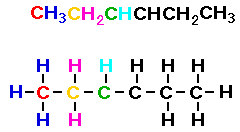
Notice that two of the carbon atoms do not have four bonds!
Yet we have accounted for all of the hydrogens shown in the
condensed formula. What do we do?? The simplest solution is to
recall that we had already identified this formula as a member of
the alkenes and we know an alkene has a double bond. So this is
where the double bond must be located.
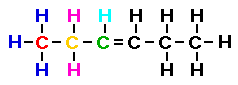
Try these examples to see how well you
understand.
Draw the Lewis structure for each of the
following compounds;
a) CH3CH2CH2CH2CH3
Answer
b) CH3CH2CH2CH2CCCH3
Answer
c) CH2CHCH2CH2CH2CH2CH2CH2CH3
Answer
d) CH3CHCHCH2CCCH2CH3
Answer
Hopefully, you will now feel better about
looking at condensed formulas and 'seeing' the Lewis structure
and the locations of double or triple bonds.