Electron Affinity
Is the energy released when an electron is added to an atom.
M(g) + 1e– ––> M–(g)
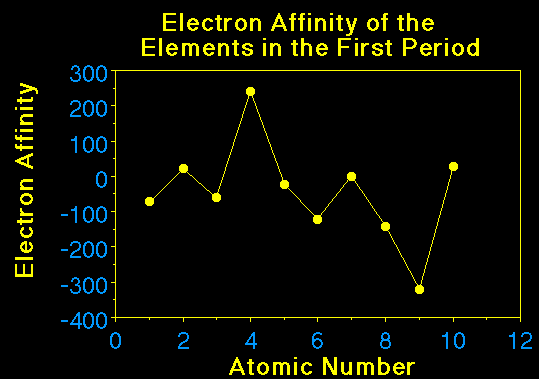
Electron affinities tend to follow the trends in ionization
energies. If the ionization energy is large, the electron
affinity is large (exothermic). It makes sense the electron
affinity should increase going across a period since the
effective nuclear charge increases. EA decreases going down a
grounp. As the distance from the nucleus of the shell the
electron is beng added to increase the energy released also
decreases.
Nitrogen is smaller than expected because it has a
half-filled subshell and adding an electron means there will be
some electron-electron repulsions incountered. So the energy
release is small, in fact it is very close to zero.