Ionic and covalent compounds
Coulomb's law
Lewis structures
Molecular geometry
Polarity
Atomic structure
Question |
Response |
Looking at a chemical formula, how do you know a compound is ionic? |
Formula contains a metal and a nonmetal, and watch out for ionic compounds with the ammonium ion. |
What do ionic compounds contain? |
Cations/positively charged ions and anion/negatively charged ions. |
Can someone define an ionic bond? |
Electrostatic attraction between oppositely charged ions. |

Question |
Response |
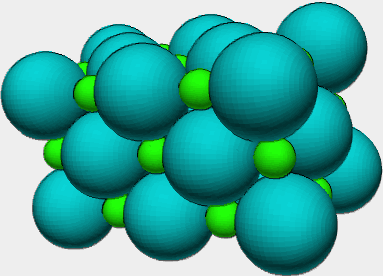
Ask students what they see in the diagram above? |
Students should respond that they see two different colored spheres; small green spheres and larger bluish colored spheres. Note it is important to begin with the simplest, obvious observations. |
Looking at the diagram of an ionic compound, who is the cation and who is the anion? |
The green sphere is the cation (loss of electrons should produce a smaller sphere for a cation.) The bluish sphere is the anion (gain of electrons should produce a larger sphere for the anion.) NOTE: my students usually claim the smaller sphere is the anion and the larger sphere the cation. |
Ask the phase of the ionic compound you are discussing/every ionic compound? |
Solid, due to the strong electrostatic attractive forces. Cations are surrounded by six anions and anions are surrounded by six cations. |
What are some melting points for an ionic compound? Very high? Very low? |
Students will not know any melting points, but they should know the melting point for an ionic compound is high. NaCl melts at 801 degrees Celsius; KCl melts at 770 degrees Celsius |
Question |
Response |
How do you recognize covalent compounds from their formulas? |
In general, the elements in the formula for a covalent compound are nonmetals. |
What kind of bonds are in covalent compounds? |
Covalent bonds. |
What is a covalent bond? |
Atoms sharing electrons as a result of the overlap of atomic or hybrid orbitals. |
Ask the phase of the covalent compounds? |
Solid, liquid and gas. |
What are some melting points and boiling points for covalent compounds? Very high? Very low? |
Students will not know many melting points or boiling points, except for water, H2O. |
Question |
Response |
Which image, F2 or HF, do you believe represents a symmetric distribution of electron density? |
The image of F2 shows a symmetric distribution of electrons. |
Which image, F2 or HF, do you believe represents a asymmetric distribution of electron density? |
The image of HF shows a symmetric distribution of electrons. |
In the image of HF what do you think the color blue and color red represent? |
H-F is a polar covalent molecule due to the dfference in electronegativity between hydrogen and fluorine. This diference is what gives rise to the polar covalent bond. So the blue color is due to the partial positive charge on hydrogen and the red color is due to the partial negative charge on fluorine. |
In the figure of HF label each atom with a symbol (δ+ or δ-) to identify the atom with partial positive charge (δ+) and the atom with partial negative charge (δ-). |
|
In the diagram of HF above an arrow (vector) has been drawn below the molecular structure. What do you think this arrow represents? Write a short explanation of what the arrow symbolizes. In your explanation use some or all of the following terms: covalent bond, nonpolar covalent bond, polar covalent bond, equal sharing of electrons, unequal sharing of electrons, electronegativity, difference in electronegativity, charge distribution, partial positive charge, partial negative charge, dipole moment. |
Question |
Response |
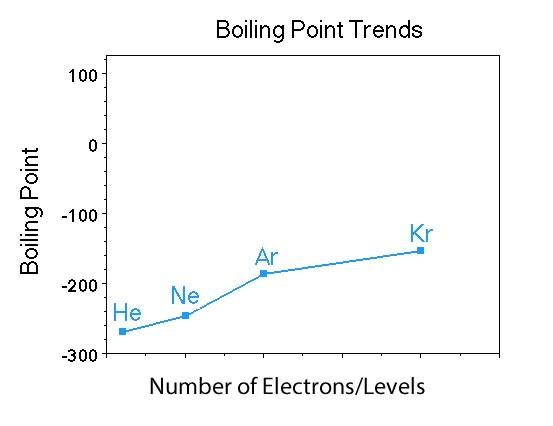
Have the students describe what they see. |
They should see the y-axis is boiling point, but also that the scale is in degrees Celsius and the x-axis is number of electrons/electron levels. So students should look at the x-axis as either the total number of electrons or the level of the valence electrons. So for He there are 2 electrons and valence electrons in the n=1 level; for Ne there are 10 electrons and valence electrons in the n=2 level; etc. |
Now ask if they see any pattern in the boiling points of the elements? |
Looking for the boiling point increases as you go down the group. Once they see the trend is for the bp to increase going down the group. Ask about the number of electrons in each of the elements. Someone is likely to say the boiling point increases as the molar mass increases, and if that is stated you need to indicate that that relationship has nothing to do with the trend in boiling points. Gravity has no connection with boiling point of substances. |
What trend do they see with electrons? |
Students should recognize that moving from helium to neon, etc the number of electrons increases. There is no explanation for that ..yet, but we will discuss it below in London Dispersion forces. Also mention that the electrons in krypton are in the n=4 level so they are very far from the nucleus compared to the electrons in helium (n=2 level), so the krypton atom has electrons that take up a large volume of space. |
One could also ask about the polarity of the elements in this group? |
All of the elements in Group VIII are nonpolar. At this point this is a factoid that we want students to have in the back of their minds. |
Question |
Response |
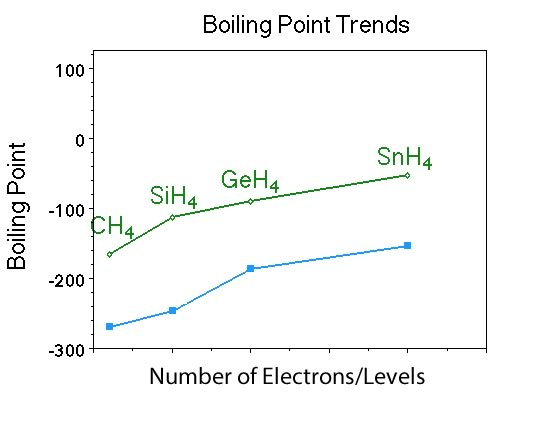
Ask students what trend do they see? |
Here students should indicate that the boiling point of the compounds increases with increasing number of electrons. They should also see that the compounds contain elements in Group IV as the central atom and the trend is for increasing boiling point with increasing electrons. Also they should note that the pattern of increasing boiling point is similar to the pattern seen in the noble gases. |
Are the members of the Group IV hydrides polar or nonpolar? |
Nonpolar, however you may need to remind them what polarity is and how important molecular geometry is for predicting polarity. Then remind them about the noble gases, were they polar or nonpolar? So polarity may help explain some of the similarity in the boiling point behavior between noble gases and Group IV hydrides. |
One could also note that all of the boiling points for the Group IV hydrides are displaced to higher temperature, but at this point, no explanation for that observation should be provided. |
When looking at the movie ask the students what do they see? (See yellow spheres that could represent a noble gas atom or a nonpolar molecule. The important point is the spherical shape represents the volume occupied by the electrons in the noble gas atom or molecule. That the shape is spherical, symmetrical, suggests that the electrons are symmetrically distributed in the atom or molecule.)So the yellow shape is representative of the electron distribution in the atom. NOTE: there is a moment in the movie where one of the yellow spheres turns many different colors, and then rotates around to reveal a look on the inside of the atom that has been sliced in half. This particular section of the movie was to reveal the symmetric distribution of electrons inside the shape. Unfortunately this brief section does not show up very well.The next change in the movie shows one of the yellow spheres in a different shape. Ask students what might have happened to cause the shape of the electron density around the atom to change? (Since the shape is representative of the electron distribution, a different shape means that the electrons for that atom are no longer symmetrically distributed. Notice how the shape rotates to reveal the electron distribution. Also to help clarify, see the δ+ (partial positive charge) and δ- (partial negative charge) that is super-imposed over the atom with the asymmetric distribution of electrons.) Now watch how the single atom with the asymmetric distribution of electrons affects the atoms near it. Notice how the side of the atom with the partial positive charge induces a partial negative charge on the side of the atom adjacent to the original atom. This happens to many of the atoms near the first atom. Then in the next instant the asymmetric distribution of electrons changes. What this model has just demonstrated is what is referred to as an instantaneous dipole (a dipole that does not last very long). A nonpolar substance has no permanent dipole, however, a nonpolar atom or molecule can have an instantaneous dipole. Instantaneous dipoles arise when the normal symmetric distribution of electrons is distorted for an instant, resulting in an instantaneous dipole. For nonpolar molecules containing atoms from the first and second period the only intermolecular attractive force that can occur is London dispersion forces, and the London dispersion force is the weakest IMAF when compared to dipole dipole forces or hydrogen bond. |
Question |
Response |
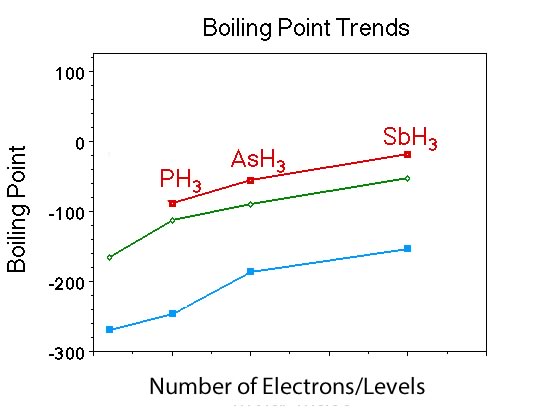
What is similar and what is different about the trend in boiling points for the Group V hydrides, compared to Group IV hydrides and the noble gases? |
Students should indicate that the boiling point of the compounds increases with increasing number of electrons just like Group IV and the noble gases. |
Ask if anyone remembers the polarity of the members of the Group V hydrides ? |
They are all polar. |
Here is a ball-and-stick model of PH3 showing where the partial positive charge and partial negative charge reside in the molecule. Since PH3 is polar and it has a permanent dipole. Molecules with permanent dipoles exhibit an intermolecular attractive force as shown below. |
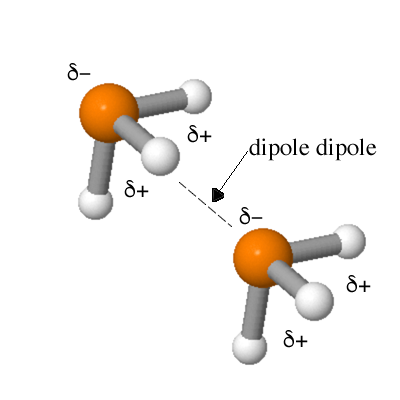 |
The dipole dipole intermolecular attractive force is found in all polar molecules. |
Question |
Response |
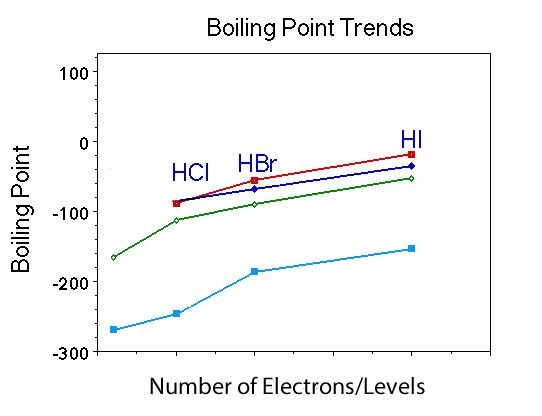
What is similar and what is different about the trend in boiling points for the Group VII hydrides, compared to Group IV and V and the noble gases? |
Students should indicate that the boiling point of the compounds increases with increasing number of electrons just like Group IV and Group V and the noble gases. However, the hydride with the fewest number of electrons, HF is similar to NH3, as it has the highest boiling point in the group. |
Ask if anyone remembers the polarity of this compounds? |
They are all polar. |
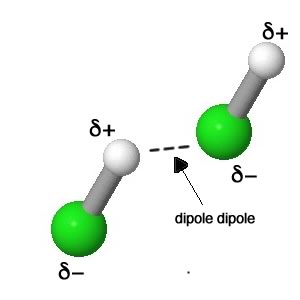 |
The dipole dipole intermolecular attractive force is found in all polar molecules. |
Can a student summarize the observations on the trend in boiling points for the four different groups (Group VIII, IV, V and VII) of substances and describe the two types of intermolecular attractive forces? |
The trend in boiling points for the substances with the higher number of electrons is the same in all four groups, independent of whether the substance is polar or nonpolar. There are two difference types of intermolecular attractive forces occuring between molecules. London dispersion forces and dipole dipole forces. LDF occur in all substances and are due to the instantaneous dipoles that result from asymmetric distributions of electrons. Dipole dipole forces occur between polar molecules and are due to permanent dipoles in the molecule. |

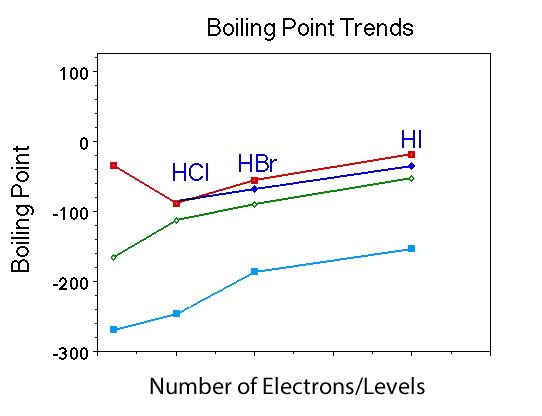
Now before asking the questions associated with Figure IV, begin with the question asking students to predict the boiling point of HF. |
Students should predict -100 C or something close. |
Oops, the actual boiling point for HF is . |
Clearly, since the boiling point for HF is considerably higher compared to the other members there must be something going on with molecules of HF that is different compared to molecules of the other Group V compounds. |
Question |
Response |
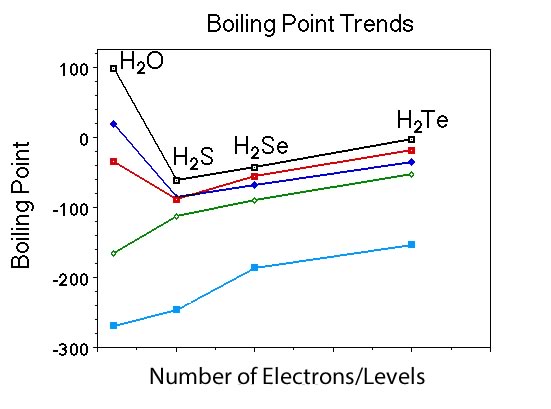
What is the formula for the members of the Group VI hydrides? |
H2O, H2S, H2Te, H2Se |
Ask if anyone can predict the trend in boiling points for each member of the group? |
Everyone should recall the boiling point for H2O, then they should predict that for H2S the boiling point drops substantuell and the then begins to increase gradually. It is not important that they know the exact boiling points for H2S, H2Te, H2Se, just that they know the pattern. |
This image of a water molecule was generated by entering 'water' in the Search field of Models 360. Then selecting, under the Display section, Molecular Electrostatic Potential, and then clicking on the radio button for MEP on isopotential surface. What we are seeing are colors that represent the electrostatic potential in a water molecules. The red color denotes a partial negative charge and the blue denotes a partial positive charge. This electrostatic potential is due to the polar bonds in H2O. The O-H bond is polar due to the difference in electronegativity between the oxygen atom and the hydrogen atom. |
|
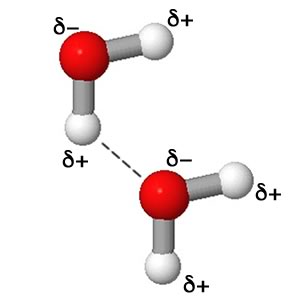 |
Here are two water molecules using a ball and stick model. Since water is polar I've added the partial positive and partial negative charges as indicated in the electrostatic potential surface as depicted using colors in the image above. The dashed line shows the electrostatic attraction (opposite charges) that could result from adjacent water molecules. Attractions between partial charges are not as strong as between full charges as experienced between cations and anions.See the simulation below for the dynamic interaction of the water molecules. Be sure at some point to select the slow motion view. |
Here are the electrostatic potential surfces for, NH3 and HF. Remember red denotes partial negative charge and blue, partial positive charge. |
Hydrogen bonds are the most important intermolecular attractive force occurring in liquid H2O, NH3 and HF. Using at least two molecules, draw the intermolecular attractive hydrogen bond that occurs in NH3 and HF. |
Students should show:
and
|
Here are the electrostatic potential surfaces for, CH2F2 and HCl. Remember red denotes partial negative charge and blue, partial positive charge. |
Here the dipole dipole interaction between molecules of CH2F2 and molecules of HCl are shown. |
________________________________________________________
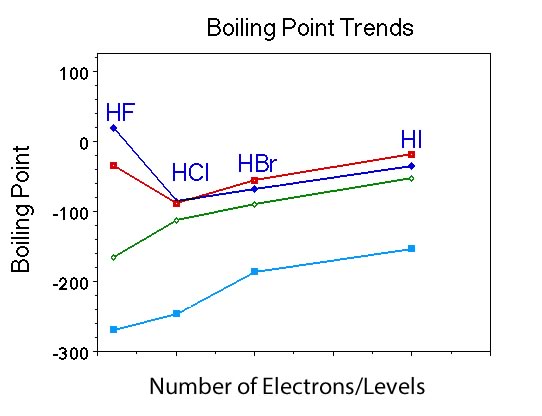
Question |
Response |
What is similar and what is different about the trend in boiling points for the Group VII hydrides, compared to Group IV and V and the noble gases? |
Students should indicate that the boiling point of the compounds increases with increasing number of electrons just like Group IV and Group V and the noble gases. However, the hydride with the fewest number of electrons, HF is similar to NH3, as it has the highest boiling point in the group. |
Ask if anyone remembers the polarity of this compounds? |
They are all polar. |
Can a student summarize the observations on the trend in boiling points for the four different groups of compounds? |
So students should recognize two different patterns. First, the trend in boiling points for the substances with the higher number of electrons is the same in all four groups. Second, there is a difference in the trend in the boiling point for the member with the least number of electrons in the four groups. For polar substances the boiling point of the hydride with the least number of electrons is higher compared to the other members, but when the compound/substance is nonpolar the boiling point of the member with the least number of electrons |
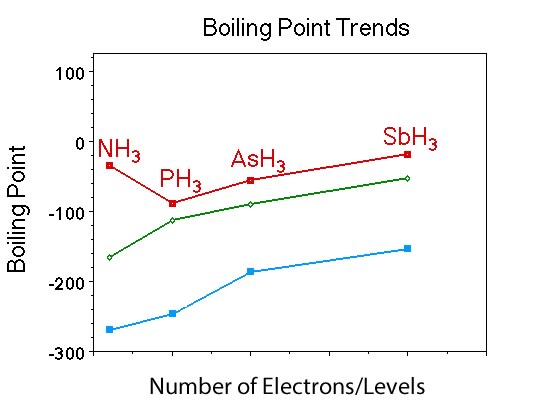
Question |
Response |
What is similar and what is different about the trend in boiling points for the Group V hydrides, compared to Group IV hydrides and the noble gases? |
Students should indicate that the boiling point of the compounds increases with increasing number of electrons just like Group IV and the noble gases. However, the hydride with the fewest number of electrons, NH3, has the highest, or nearly the highest boiling point in the group. |
Ask if anyone remembers the polarity of the members of the Group V hydrides ? |
They are all polar. |
Question |
Response |
Now before asking the questions associated with Figure III, begin with the question asking students to predict the boiling point of NH3. |
Students should predict -110 C or something close. They will make this prediction becasue they are following a pattern that was observed in the Group IV hydrides and the Group VIII elements. |
So this is the first introduction to something new, some kind of new interaction that is occuring between NH3 molecules. |
Clearly, since the boiling point for NH3 is considerably higher compared to the other members there must be something going on between the molecules of NH3 that is different compared to what is going on between the molecules of the other Group V compounds. |
At this point it may be worthwhile to discuss what happens when the liquid phase of a pure substance changes to the gas phase. A general chemical equation describing this change would be;A(l) --> A(g) |
The point of bring up the chemical equation of the phase change is to get the students to begin thinking about what must happen for particles of A, in the liquid phase to |
Question |
Response |
What is similar and what is different about the trend in boiling points for the Group VII hydrides, compared to Group IV and V and the noble gases? |
Students should indicate that the boiling point of the compounds increases with increasing number of electrons just like Group IV and Group V and the noble gases. However, the hydride with the fewest number of electrons, HF is similar to NH3, as it has the highest boiling point in the group. |
Ask if anyone remembers the polarity of this compounds? |
They are all polar. |
Can a student summarize the observations on the trend in boiling points for the four different groups of compounds? |
So students should recognize two different patterns. First, the trend in boiling points for the substances with the higher number of electrons is the same in all four groups. Second, there is a difference in the trend in the boiling point for the member with the least number of electrons in the four groups. For polar substances the boiling point of the hydride with the least number of electrons is higher compared to the other members, but when the compound/substance is nonpolar the boiling point of the member with the least number of electrons |