![]() Pre-lab Questions
Pre-lab Questions
![]() Experiment
Experiment
![]() Post-lab Questions
Post-lab Questions
EXPERIMENT 6: CONDUCTIVITY
![]()
(a) phosphoric _________;
(b) perchloric _________;
(c) nitric _________;
(d) sulfuric _________;
(e) hydrochloric _________;
(f) acetic _________.
(a) calcium hydroxide _________;
(b) potassium hydroxide _________;
(c) sodium hydroxide _________;
(d) ammonia _________.
(a) potassium chromate _________;
(b) potassium sulfate _________;
(c) copper (II) nitrate _________;
(d) calcium carbonate _________;
(e) potassium iodide _________;
![]()
![]()
EXPERIMENT 6: CONDUCTIVITY
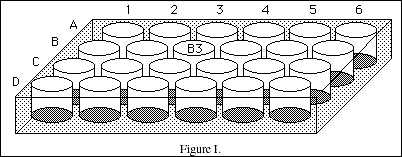
In this experiment (and again in Experiment 9) you will use a piece of equipment called a well plate shown in Figure I. This piece of equipment is made of clear plastic and contains wells used to hold solutions. Each well can be identified using a combination of a letter and a number. In the figure, well B3 (2nd row-3rd column) is labeled. A particular well will be indicated by a letter (row) and a number (column). In this experiment you will use a 96-well plate. The wells are small and only a few drops of reagent will be needed. Doing experiments on a 'microscale' is very economical and considerably safer than large scale experiments.
![]()
![]()
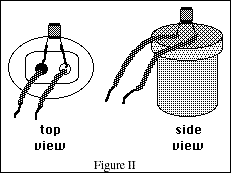
The conductivity apparatus used in this experiment consists of a 9-volt battery inside a 35 mm
plastic film container. A light-emitting-diode (LED) has been wired to the battery. Two wires
(electrodes) are attached to the LED so that if the electrodes are placed in a solution which conducts
electricity the LED will glow at a particular intensity. The best way to observe the light intensity after
immersing the electrodes into a conducting test solution is to view the LED from the top, not the side.
![]()
![]()
EXPERIMENT 6: CONDUCTIVITY
![]() Top
Top
| EQUIPMENT: | |
| beaker, 50 mL ........................ 1 | petri dish .............................1 |
| conductivity apparatus ............... 1 | plate, 96-well........................1 |
| dropper ................................ 1 | spatula or microspatula.............2 |
| paper towels........................... several | watch glass ..........................2 |
Check the conductivity apparatus using a test solution. Place 4 drops of the test solution in H12 and insert the electrodes of the conductivity apparatus into the test solution. Check that the LED (light emitting diode) glows brightly. When viewing the LED, look down from above the LED rather than from the side. It may also help to slightly darken the room. If the conductivity apparatus works, remove the electrodes from the solution and wash the electrodes with deionized water and dry with a paper towel. It is important to wash the electrodes with deionized water following each measurement. Try not to immerse the electrodes so deep that solution leaks underneath the plastic sheath covering the wires. If this happens clean the electrodes carefully to prevent erroneous observations.
Using the 96-well plate and a dropper, fill A1 with tap water and introduce the electrodes to a depth of about 5 mm. Note whether the LED glows brilliantly, faintly, or not at all.
![]()
Try immersing the electrodes more and more deeply into the tap water. Record your
results.
![]()
NOTE: For each of the tests that follow, you should immerse the electrodes to
approximately the same depth.
Dry the electrodes. In A3, test deionized water using the conductivity apparatus. Does the LED glow brilliantly, moderately, faintly, or not at all?
![]()
Test dry sucrose (C 12H 22O11 ) with the conductivity apparatus. Use a microspatula to half-
fill A7. Be careful not to spill sucrose in the wells surrounding A7 and be sure the
electrodes are clean and dry before testing the sample.
![]()
![]()
With the electrodes still in contact with the solid sucrose in A7, add a few drops of deionized water and observe LED. Record your observations.
![]()
In A9, test dry sodium chloride (NaCl) with the conductivity apparatus. Use a dry microspatula to
place the NaCl into the well. Be sure the electrodes are dry before testing the sample.
![]()
With the electrodes still in contact with the dry sodium chloride in A9, add a few drops of
deionized water. Record your observations.
![]()
Clean and dry the conductivity apparatus electrodes. Place 4 drops of KI solution in A11 and then
test the conductivity of KI. Record your observations.
![]()
Clean and dry the conductivity apparatus electrodes. Test a sample of Pb(NO3)2 solution in C1.
Record your observations.
![]()
Place a small sample of solid lead nitrate on a watch glass. On a second watch
glass, place a sample of solid potassium iodide.
Briefly describe the initial appearance of the dry potassium iodide and lead nitrate solids.
![]()
Clean a Petri dish. Cover the bottom of the dish with a thin layer of deionized
water. Use a spatula to carefully place a few crystals of lead nitrate into the water close to
one side of the Petri dish. Try not to agitate the water when adding the solid. Use a clean
spatula to carefully add a few crystals of potassium iodide to the opposite side of the Petri
dish. Do not bump the Petri dish. It is important that the water not be agitated during the
experiment. Watch what happens.
![]()
![]()
Draw the arrangement of the Petri dish and the samples of lead nitrate and potassium iodide in the space below. Draw a second picture showing what happened as time passed.
![]()
Briefly describe your observations of what happened after you placed the solids in the
dish.
![]()
Do not continue experimenting until your instructor has completed the group discussion to
clarify the experiment just performed. Following the discussion explain your observations.
In your explanation include the use of the following terms: anion, cation, electrolyte,
precipitate, soluble, hydration, and electrical conductivity.
![]()
![]()
Explain your observation of the conductivities of deionized water and tap water.
![]()
Explain your observations of the conductivities of solid sucrose and of sucrose solution.
![]()
Explain your observations of the conductivities of solid sodium chloride and of sodium
chloride solution.
![]()
Explain the difference in the conductivities of the sodium chloride solution and sucrose
solution.
![]()
![]()
EXPERIMENT 6: CONDUCTIVITY
EQUIPMENT:
beaker, 50 mL ........................ 1
conductivity apparatus ............... 1
dropper ................................ 1
paper towels........................... several
plate, 96-well ......................... 1
Place four drops of 0.1 M hydrochloric acid (HCl) in C3, four drops of 0.1 M acetic acid (HC2H3O2) in C5, and four drops of 0.1 M sulfuric acid (H2SO4) in C7. Compare the conductivities of the three solutions by observing the intensity of the LED, for example, no glow (non conductor), faint (poor conductor) or brilliant (good conductor). Be sure to clean and dry the elecrodes after each use. Classify each acid as either a nonelectrolyte, weak electrolyte or strong electrolyte. Identify the ions present in each solution which account for the conductivity.
![]()

Place four drops of 0.1 M sodium hydroxide (NaOH) in C9 and four drops of 0.1 M ammonia (NH3) intensity of the LED. Be sure to clean and dry the electrodes after each use. Classify each in C11. Compare the conductivities of the two solutions by observing the
base solution as either a nonelectrolyte, weak electrolyte or strong electrolyte. Identify the ions that are present in each solution which would account for the conductivity.
![]()

![]()
![]()
Place four drops of 0.1 M sodium acetate (NaC2H3O2) in E1, four drops of 0.1 M sodium chloride (NaCl) in E3, four drops of 0.1 M ammonium acetate (NH4C2H3O2) in E5, four drops of 0.1 M ammonium chloride (NH4Cl) in E7. Use the conductivity apparatus to check each solution. Be sure to clean and dry the electrodes after each test. Record your results. Classify each solution as either a nonelectrolyte, weak electrolyte or strong electrolyte. Identify the ions that are present in each solution which would account for the conductivity.
![]()
![]()
Place four drops of methanol (CH3OH) in E9 and four drops of ethanol (C2H5OH) in E11.
Compare the conductivities of the two solutions by observing the intensities of the LED,
for example, no glow (non conductor), faint (poor conductor) or brilliant (good
conductors). Be sure to clean and dry the electrodes after each use. Classify each
compound as either a nonelectrolyte, weak electrolyte or strong electrolyte. Identify the
ions that are present in each solution which would account for the conductivity.
![]()

![]()
Consider the following sample set of observations and the equation that results. Jennifer
Redleg, an aspiring chemistry student, tested the conductivity of a 0.1 M nitric acid
solution (HNO3) and found that the LED glowed brightly. Jennifer concluded that the
HNO3 is a strong electrolyte. To demonstrate her knowledge of the ions formed in the
solution Jennifer wrote the following equation:
HNO3(aq)
 H+(aq) + NO3-(aq) .
H+(aq) + NO3-(aq) .
![]()
![]()
The equation can be interpreted the following way: An aqueous solution of HNO3 contains the hydrated ions H+ and NO -3 . Jennifer realized that she could write HNO3(aq) or H+(aq) and NO3-(aq) to indicate an aqueous nitric acid solution. Write a similar chemical equation which indicates the species that are in solution for each of the compounds whose conductivities were measured.
![]()
![]()
You have now collected data on a variety of substances. You have classified each solution as a nonelectrolyte, weak electrolyte or strong electrolyte. The terms strong electrolyte, weak electrolyte, or nonelectrolyte are used to summarize the experimental observations and refer to the ability of the compound to conduct electricity. In the case of the strong and weak electrolytes you identified the ions in solution that were responsible for the solutions observed ability to conduct electricity.
List the solutions that are strong electrolytes.
![]()
List the solutions that are weak electrolytes:
![]()
List the solutions that are nonelectrolytes:
![]()
Based on your observations of the above 14 solutions, what classes of compounds are
strong electrolytes when dissolved in water? Weak electrolytes? Nonelectrolytes? (Note:
See the preparatory questions you answered at the beginning of the experiment .)
![]()
![]()
EXPERIMENT 6: CONDUCTIVITY
| EQUIPMENT: | |
| laboratory balance .................... 1 | paper towels .........................3 |
| beaker, 50 mL ........................ 2 | plate, 96-well........................1 |
| capillary tube.......................... 1 | ring stand ............................2 |
| conductivity apparatus ............... 1 | spatula................................1 |
| dropper ................................ 1 | stopper, 1-hole #6 ..................1 |
| flask, Erlenmeyer, 250 mL.......... 1 | trough or pan ........................1 |
| glass bend ............................. 1 | tubing,1' rubber.....................1 |
| glass tubing ........................... 1 | utility clamps ........................2 |
| graduated cylinder, 50 mL .......... 1 | weighing paper......................3 |
You will assemble an apparatus such as that shown in Figure 1. Start by filling the trough and the graduated cylinder with deionized water. Invert the graduated cylinder into the trough. After you have inverted the graduated cylinder be sure that it remains full of water. Fit the Erlenmeyer flask with a 1-holed rubber stopper. Your instructor will demonstrate how to insert glass tubing into a rubber stopper. Follow these instructions carefully. Please ask if you have questions. The procedure is also demonstrated on the pre-lab video tape for Experiment #1. Set up your apparatus as shown in Figure I. Complete your setup and be sure that all parts fit snugly so that no gas can escape. Have your instructor check your apparatus before you begin the experiment.
Apparatus Checked ________________
![]()
![]()
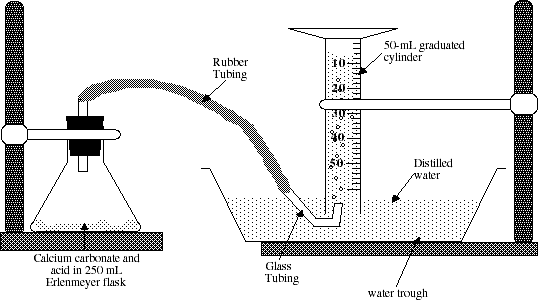
Figure I.
Measure approximately 2 grams of powerded calcium carbonate (CaCO3) onto a small
piece of paper. Be sure the sample texture and particle size is uniform. Obtain 30 mL of
the 1 M HCl in a small beaker. After the instructor has checked your apparatus, and you
are ready, add the acid to the Erlenmeyer flask. Then add the calcium carbonate to the acid
in the Erlenmeyer flask, quickly stopper the flask and collect the escaping gas in the
graduated cylinder. Note the time required to collect 20 mL of gas. The acid may react
with CaCO3 very rapidly, generating the 20 mL of gas quickly. You may wish to indicate
the time as less than a second. Clean and rinse the flask and repeat the experiment for the
other two acids (be sure to use 1 M HC2H3O2
and 0.5 M H2SO4). It is not necessary to
try to time these reactions. Simply compare and note the relative rate of evolution of gas.
(Hint: Two of these should be about the same and the third noticeably different.)
Rate of evolution of 20 mL of gas
1 M HCl....1 M H(C2H3O2)....0.5 M H2SO4
____________..__________..__________
On the basis of your experimental data arrange the acids in the order of decreasing strength.
____________ ____________ ____________
Write the reaction that occurs in the Erlenmeyer flask for each acid.
Why was it important to have the three samples of CaCO3 match in texture and particle size?
Remove the electrodes of the conductivity apparatus from the solution of calcium
hydroxide. Blow through a capillary tube into the solution and then measure its
conductivity again. (Note: Be sure you are wearing your goggles). Keep repeating the
procedure. Clean and dry the electrodes between measurements. Measure the conductivity
of the solution and record your observations. Be sure to note any changes in the
appearance of the solution.
EXPERIMENT 6: CONDUCTIVITY
Observe the color of 0.1 M solutions of the salts listed below and record the formula and
color for both the cation and the anion. (If a solution is colorless, the ions it contains must
also be colorless. The color of an ion is independent of the color of any other ions in the
solution.)
Cite two methods you could use to test for ions in solution.
Add a crystal of iron(III) nitrate, Fe(NO3) 3
to one side of a Petri dish containing deionized
water and then add a few crystals of ammonium
thiocyanate, NH4SCN to the other side.
Describe what happens. Include a picture. 4SCN, to the other side.
The answers to the following problems must accompany your laboratory report.
(a) HNO3(aq) and NaOH(aq)
![]()
Record your observations for each acid.
Obs. #16
![]()
![]()
Reactions
![]()
How do you explain the difference in the time required to generate 20 mL of gas?
Expl. #7
![]()
![]()
Expl. #8
![]()
Why was 0.5 M H2SO4 used instead of 1 M H2SO4?
Expl. #9
![]()
The concentration of hydrogen ion, H+, in 1.0 M HC2H3O2 is 0.0042 M. How would the
rate or reaction between 1.0 M HC2H3O2 and CaCO
between 0.0042 M HCl and CaCO ? Explain. 3 compare with the rate of reaction
3
Expl. #10
![]()
Place four drops of saturated (0.1 M) calcium hydroxide (Ca(OH)2) in G7 and test its
conductivity. Make sure the well is dry and free of contamination. Note: Be sure the
sample of saturated calcium hydroxide you use is clear and colorless. If the sample is
cloudy, check with the instructor.
Obs. #17
![]()
![]()
Obs. #18
![]()
Write a chemical reaction which describes what is occurring when you exhale into a saturated
solution of calcium hydroxide.
Reaction
![]()
Explain your observations of the conductivity of the solution.
Expl. #11
![]()
![]()
EQUIPMENT:
beaker, 50 mL ........................ 1
paper towels ..................... several
conductivity apparatus ............... 1
plate, 96-well........................2
dropper ................................ 1
spatula................................1
![]()
PART IV: Chemical Reactions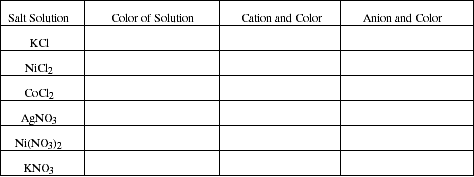
![]()
Place two drops of 0.1 M AgNO3 with four drops of 0.1 M NiCl
happens, then test the conductivity of the solution. 2 in G9. Observe what
Obs. #19
![]()
Identify the precipitate (if any) and the ions present in solution. Explain how you arrived at
your conclusions.
Expl. #12
![]()
Write an ionic equation for the reaction.
Reaction
![]()
![]()
Expl. #13
![]()
Ions that remain unreacted in a solution are called spectator ions. A net ionic equation can be
obtained by algebraically cancelling all the spectator ions. Write the net ionic equation for the
above reaction.
Reaction
![]()
Place two drops of 0.1 M AgNO3 and four drops of 0.1 M CoCl2 in G11. Observe what
happens. Test the conductivity of the solution. (Be sure to clean the electrodes of the
condutivity device.)
Obs. #20
![]()
Write the ionic and net ionic equations for the reaction.
Reaction
![]()
![]()
Obs. #22
![]()
Explain what must be happening in the solution to account for your observations.
Expl. #14
![]()
Write the ionic and net ionic equations for the reaction.
Reaction
![]()
![]()
![]()
(b) KNO3(aq) and NiCl2(aq)
![]()
(c) AgNO3(aq) and KCl(aq)
![]()
(d) Ni(NO3)2(aq) and AgNO3(aq)
![]()
(e) H2SO4(aq) and CaCO3(aq)
![]()
(f) HCl(aq) and Na2CO3(s)
![]()
![]()
![]() Aqueous ammonia, NH3(aq), and acetic acid, HC2H3O2(aq), solutions of equal
concentrations, conduct electric current equally well. Explain why the addition of one
solution to the other results in a substantial increase in electrical conductivity.
Aqueous ammonia, NH3(aq), and acetic acid, HC2H3O2(aq), solutions of equal
concentrations, conduct electric current equally well. Explain why the addition of one
solution to the other results in a substantial increase in electrical conductivity.
![]() Ammonium sulfate and barium hydroxide solutions are each very good conductors.
However, when equal volumes of solutions of equal concentrations are mixed, a
dramatic decrease in conductivity is observed. Explain.
Ammonium sulfate and barium hydroxide solutions are each very good conductors.
However, when equal volumes of solutions of equal concentrations are mixed, a
dramatic decrease in conductivity is observed. Explain.
![]()
![]()
(a)
HClO4
(b)
Ca(NO3)2
(c)
NH2CONH2 (urea)
(d)
HBr
(e)
H3PO4
(f)
(NH4)2CO3
(g)
PbCl2
(h)
KOH
(i)
C3H5(OH)3 (glycerol)
(j)
PbI2
(k)
CH3CH2CH2OH
![]()
Contents
![]() Return to
Return to![]() Index of Experiments
Index of Experiments