![]() Pre-lab Questions
Pre-lab Questions
![]() Experiment
Experiment
![]() Post-lab Questions
Post-lab Questions
EXPERIMENT 4: ACID-BASE TITRATION

![]()
(b) Why are air bubbles in the buret tip a possible source of error in a titration
experiment? How do you remove air bubbles from the buret tip?
![]()
c) In the picture BELOW are sections from two
burets. One is labeled 'Initial', the other
'Final'. Draw the correct level of the
solution in each buret, if the initial volume
is 1.0 mL and the final volume is 6.5 mL.
Be sure to draw the meniscus properly.
What is the volume of the solution
delivered?
![]()
![]()
![]()
(d) Why is not recommended to have an initial buret volume of 0.00 mL?
![]()
(e) What indicator is used in this experiment? Describe the color change of the indicator.



(a)
![]()
(b)
![]()
![]()
EXPERIMENT 4: ACID-BASE TITRATION
![]() Top
Top
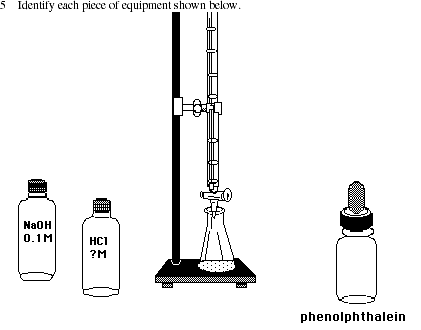
| EQUIPMENT: | |
| laboratory balance.......................1 | flask, Erlenmeyer, 125 mL........ 1 or 2 |
| beaker, 100 mL .........................2 | pipet, volumetric, 20 mL ..........1 |
| beaker, 250 mL .........................1 | ring stand............................2 |
| bulb, pipet or pipet pump ..............1 | spatula ...............................1 |
| buret clamp ..............................1 | wash bottle ..........................1 |
| buret, 25 mL or 50 mL.................1 | white paper, sheet ..................1 |
| cylinder, graduated, 50 mL............1 | white paper, 1"x3" strip ...........1 |
| cylinder, graduated, 100 mL ..........1 |
![]()
In the first part of the experiment you will determine the molarity of an aqueous
solution of sodium hydroxide (NaOH) to 3 significant figures. The concentration of the
NaOH is known to be approximately 0.1 M.
(HAZARDS: Concentrated solutions (6-12 M) of NaOH can cause severe skin burns and permanent damage to eyes. Dilute solutions of NaOH should be treated as hazardous chemicals.)
You will react the NaOH solution with a known amount of the solid acid potassium hydrogen phthalate (KHP). The formula for KHP is KHC8H4O4, and it has one ionizable proton. KHP is a primary standard reagent. That means that it is available in a very pure form (> 99.9% pure) and it can be used to very accurately determine the concentration of another substance of unknown concentration. Write the ionization reaction for KHP.
![]()
Write the equation for the reaction that will occur when NaOH is added to KHP.
![]()
![]()
Weigh, to the nearest milligram, between 0.34 g and 0.36 g of KHP into a 125 mL flask and record the mass.

Add 40 mL of deionized water to the 125 mL flask, and swirl the mixture until all of the KHP has dissolved. Add 3 to 4 drops of phenolphthalein indicator. Obtain 100 mL of the NaOH solution from the instructor. Prepare a buret by rinsing it first with water and then with a few milliliters of the NaOH solution to be standardized. Discard the rinse solution and fill the buret with the NaOH solution. Before proceeding, calculate the approximate volume of 0.1 M NaOH needed to neutralize the amount of KHP in Obs. #1.
![]()
Place a small strip of white paper behind the buret at the level of the solution to provide
contrast for the mensicus. Read and record the initial level of the sodium hydroxide solution in the
buret to ±0.01 mL (Obs. #2). Place a sheet of white paper below the buret. Set the flask on the
paper. Titrate the solution. Stop frequently to swirl the contents of the flask. If any drops of
sodium hydroxide cling to the walls of the flask, rinse them down with a stream of water from the
wash bottle. Continue adding sodium hydroxide solution dropwise until a pale pink color which
persists for at least 30 seconds appears throughout the solution.. Record the final level of the
solution in the buret (Obs. #2).
![]()
![]()

![]()
Calculate the molarity of the NaOH. (Show all calculations.)
![]()
Perform a second titration as a check.
![]()

![]()

Calculate the molarity of the NaOH. (Show all calculations.)
![]()
![]()
If the values of the first two titrations agree within 5 %, they should be averaged. If they do not, a third titration should be performed. Calculate the average value of the molarity of the NaOH. Use this average value in all subsequent calculations.
![]()
Now that you know the concentration of the NaOH, you can determine the
concentration of an unknown acid solution. For this experiment an HCl solution of
unknown concentration has been prepared.
(HAZARDS: Concentrated solutions (6-12 M) of HCl can cause severe skin burns and permanent damage to the eyes. Inhalation can cause coughing and choking. Dilute solutions of HCl should be treated as hazardous. If you should come in contact with either solution, rinse the area liberally with water.)
Write the overall neutralization equation for the reaction of NaOH with HCl.
![]()
![]()
Obtain 50 mL of the unknown hydrochloric acid solution from your instructor and record the unknown number.
Rinse both the 125 mL flask and a 20 mL pipet with deionized water. Rinse the pipet using a small volume of the unknown acid solution. Pipet 20.00 mL of unknown acid into the 125 mL flask. Add about 20 mL of deionized water and a few drops of phenolphthalein. Complete the titration and record the volume of NaOH added below. Repeat the titration.
Volume of unknown HCl solution _________ mL

![]()
Calculate the concentration of the unknown HCl solution. (Show your work!)
![]()
![]()
![]()
![]()
Titration Tips
![]()
![]()
Bubbles in the buret tip can usually be removed by tapping the side of the buret while the stopcock is open. This is best done over a sink!
![]()
![]()
There are lots of acid-base indicators you could use for your titration. Phenolphthalein is a good all around choice because it turns from colorless to colored (it is much easier for the human eye to distinguish than changes from one color to another) and because of the pH range over which it changes. Consult your text or other chemistry reference book if you are interested in using another indicator.
The color of a solution can interfere with that of the indicator. If your solution is colored, run a few trials to see what the solution and indicator look like at various pH values before you start to titrate. Sometimes diluting a colored solution with deionized water will make the indicator more visible.
It is easier for the eye to distinguish a change from colorless to colored than from colored to colorless, so if phenolphthalein is your indicator it is best to titrate the acid solution with the base (although in theory you could titrate either way).
While titrating, it is a good idea to wash down the walls of the flask with deionized water so that all of the titrant gets into the reaction mixture. Addition of small to moderate amounts of deionized water will not change the results of your titration.
Be consistent when reading your buret. The same eye that makes the initial reading should also make the final reading. Any absolute errors will then subtract out. This does not mean, however, that one lab partner must do all the titrations - it just means finish the ones you start!
The desired end point in a phenolphthalein titration is "A pale pink tint that persists for 30 seconds". If you are not sure if you have reached this point, record the volume of the buret, then add one more drop of base. If the solution turns dark pink or red, you were right. If not, keep titrating. As a rule of thumb, endpoints that look like pink lemonade or paler are probably satisfactory - endpoints that look like cherry kool-aid or darker will probably have to be repeated.
![]()
![]()
Some people like to do a quick "throw away" titration. They add the titrant very rapidly up to and a little beyond the endpoint to get a general idea of how much titrant will be required. Then they can perform titrations more quickly by adding titrant rapidly until the endpoint is near and then switch to adding dropwise.
If your titration seems to be taking a lot of titrant (more than 1 or 2 burets full) you may have one of the following problems: a) forgot to add indicator b) titrating with wrong solution c) solution being titrated is too concentrated
In general, 3 consistent titrations are required to determine the concentration of an unknown.
Titration is a skill that takes a lot of practice to perfect. Be patient and don't give up if you want to develop a skill that will serve you well in many chemistry labs to come.
It almost always takes less time to do an experiment once, slowly and carefully, than to do it as fast as you can over and over until you get it right.
Do not waste your time trying to line up some initial volume such as 0.10 or 1.00 in the buret. Read it to + 0.01 mL wherever it happens to fall. Never start with a volume of 0.00 mL - this is the least accurate measurement on the scale because there is nothing above it to use as a reference. If your titration requires more than 1 buret full of titrant, be sure to record a final volume before the solution level passes the end of the scale and then refill the buret and start titrating again.
If, for some reason, a direct titration procedure does not work well, there is a technique called "back titration" that may solve the problem. If you are interested in performing a back titration, you can look up procedures in the library. Most text books on Analytical Chemistry give a good description of the process.
![]()
Contents
![]() Return to
Return to![]() Index of Experiments
Index of Experiments