![]() Pre-lab Questions
Pre-lab Questions
![]() Experiment
Experiment
![]() Post-lab Questions
Post-lab Questions
EXPERIMENT 1: CLASSIFICATION AND SEPARATION OF MATTER

as a dictionary, in addition to the text of this experiment.)
(a) homogeneous mixture
![]()
(b) heterogeneous mixture
![]()
(c) solution
![]()
(d) physical change
![]()
(e) chemical change
![]()
(f) filtrate
![]()
![]()
(g) chromatography
![]()
(h) relative rate of movement, Rf
![]()
(i) distillation
![]()
(j) fractional distillation
|
differ? How are they similar?
![]()
![]()
|
![]()
Given a powdered mixture containing sulfur, carbon and iron, devise a stepwise procedure
which would separate all the components. (Note: Carefully describe the separation
procedures you use and how you would obtain the pure solid components. Carbon
disulfide is extremely flammable; do not use an open flame in your scheme. Carbon
disulfide is also very poisonous.)
![]()
![]()
EXPERIMENT 1: CLASSIFICATION AND SEPARATION
OF MATTER
![]() Top
Top
| EQUIPMENT: | |
| balance...................................1 | graduated cylinder, 10 mL ...1 |
| beaker, 100 mL.........................1 | ring clamps.....................2 |
| Bunsen burner ..........................1 | ring stand.......................2 |
| cork to fit 13 x 100 mm test tube .....1 | rubber policeman ..............1 |
| evaporating dish ........................1 | spatula ..........................1 |
| filter paper........................... 1 circle | stoppers to fit test tube........3 |
| funnel, polyethylene ...................1 | test tube, 13 x 100 mm ...... 12 |
| glass stirring rod........................1 | wash bottle .....................1 |
If you have not already done so, please read the foreword to this manual before beginning the experiment.
In this experiment some properties of three substances will be studied. Based upon the observed properties a scheme must be developed to separate the components of a mixture.
Before beginning the experiment, several techniques which will be useful in completing the separation of a mixture will be demonstrated by your instructor. Use the space below to record important notes.
![]()
![]()
Obtain approximately 0.1 g each (do not weigh) of sand, NaCl (sodium chloride) and CaCO3equivalent to 0.1 g - it is not necessary to weigh the solids on the balance at this point in the (calcium carbonate) in 13 x 100 mm test tubes. Use the approximate volume that is
procedure. Describe the appearance of each of the solids.
![]()
To each test tube containing one of the solids add 5 mL of deionized water. Stopper and
shake. Record your observations.
![]()
If a change occurs in Obs. #4 - 6, is it a physical or chemical change? ________________ If a
chemical changes occurs, write the chemical equation.
![]()
![]()
Clean the three 13 x 100 mm test tubes and obtain another 0.1 g sample of each solid. To each test tube containing one of the solids add 5 mL of dilute (6 M) HCl (hydrochloric acid). Caution: Dilute hydrochloric acid is corrosive. Be very careful when adding this solution to the solids. Do not stopper or shake these test tubes. Record your observations.
![]()
If a change occurs in Obs. #7 - 9, is it a physical or chemical change? ________________ If a
chemical changes occurs, write the chemical equation.
![]()
Summarize your observations (#4 - 9) in a tabular form similar to that used in Problem 4 of the
Pre-lab.

![]()
![]()
If you had a mixture of sand and salt (NaCl), briefly describe how you would separate and determine the composition of the mixture. (Note: Use the decanting and filtering techniques demonstrated by your instructor.)
![]()
If you had a mixture of sand and calcium carbonate (CaCO3), briefly describe how you
would separate and determine the amount of each the mixture. (Note: Use the decanting and
filtering techniques demonstrated. One of the components of the mixture may be converted into
another compound during this procedure.)
![]()
![]()
Shown below is an incomplete flow chart for the separation of a mixture of the three solids
(sand, NaCl and CaCO3). Based on your observations, complete the flow chart by filling in the
blank spaces (A, B, and C) with the name of the solid obtained. Due to a chemical reaction, one
of these will be different than the original solids in the mixture.
Using the analytical balance, weigh your evaporating dish to the nearest 1 mg and record
the mass.
Calculate the mass of the original unknown sample.
Add 5 mL of deionized water to the unknown sample in the evaporating dish. Stir the
mixture carefully to dissolve Component A. What is the identity of Component A?
When the evaporation is complete, cool the dish to room temperature. Weigh the
evaporating dish and the solid residue. Record the mass.
Mass of cool evaporating dish
and solid residue (Component A) ............ ______________
Dispose of the solution.
Rinse the solid Component B with a small amount of deionized water from the wash bottle.
Carefully lift the filter paper and Component B into a beaker and leave them to dry until the next
laboratory. What is the identity of Component B?
When the filter paper and Component B are dry, weigh them together. Determine the mass of
components B and C.
Calculate the percentage of each component in the original sample. (Show your work in the
space provided. All work must be shown to receive credit.)
% of CaCO3 in original sample...............______________ %
EXPERIMENT 1: CLASSIFICATION AND SEPARATION
OF MATTER
Chromatography is a technique used to physically separate the components of a mixture.
Historically, chromatography was used to separate mixtures of colored components, such as
pigments or dyes. The word chromatography means "graphing colors" in Greek. Today,
however, chromatographic techniques are not limited to colored samples. Almost any type of
mixture can be separated using chromatography, even if only a tiny amount is available for testing.
In addition to separation, chromatographic methods can sometimes provide information about the
percent composition of the mixture and the identity of the components.
There are several different types of chromatography, but all have certain characteristics in
common. Mixtures are separated by distributing their components between two phases - one
stationary and one mobile. The mobile phase carries a sample of the mixture through the stationary
phase. The components of the mixture are separated by the differences in their attractions to the
two phases. Components that are strongly attracted to the mobile phase move more rapidly than
components that are strongly attracted to the stationary phase. If the stationary phase (often called
the chromatography column) is long enough, the components will be separated completely due to
this difference in the rate of travel. The separation is very much like a group of runners in a race.
If the race is very short, all of the runners will cross the finish line within a few seconds of one
another. If the race is very long, a marathon for example, the runners will spread out a great deal.
The last runner may cross the finish line hours after the first. Some runners may have to stop
along the course and not reach the finish line at all. The same is true for the components of a
mixture in a chromatographic separation. Unfortunately, it is seldom possible to be absolutely
certain that all of the components of the mixture have been separated. Two components might "tie
in the race." Thus, chromatography always tells the minimum number of components in a
mixture. This may or may not equal the total number of components.
The materials used for the stationary and mobile phases can each be in any of the three
states of matter - solid, liquid, or gas. Particular materials are selected in order to maximize the
separation of particular mixtures. The shape and other properties of the different component
molecules determine the attractions the molecules will feel toward substances used in the two
phases. In general, it is important that the molecules of the stationary and mobile phases have
somewhat different properties in order for a good separation to occur. Very often, the stationary
and/or mobile phases used to accomplish a desired separation must themselves be mixtures.
The first, and perhaps the simplest, type of chromatography is called column
chromatography. In column chromatography, a glass or plastic tube, open at both ends, is packed
with porous particles of an inert (nonreactive) solid such as alumina or silica. The solid is held in
the tube by a filter that will allow only liquids to pass through. A sample of the mixture to be
separated is placed on the top of the column and washed down the column with a liquid, usually a
solution of organic liquids, sometimes including water. The solid in the tube (or column) is the
stationary phase and the liquid (or solvent) is the mobile phase. The solvent dissolves the sample
mixture and carries it through the column around all of the tiny particles. Component molecules
attracted to the molecules of the solid become adsorbed onto the surface of the stationary particles
and travel through the column very slowly. Component molecules which are attracted to the
solvent move rapidly down the column. The solvent is collected as it passes out the bottom of the
column. If the components of the mixture are colored, they can be seen traveling down the column
in bands or rings of color. The colored components can be collected in separate containers as they
exit the column with the solvent. It is often necessary to change the composition of the solvent
during the chromatographic separation because some components are so strongly attracted to the
stationary phase that they would never leave unless a liquid to which they were more strongly
attracted is added.
A technique very similar to column chromatography is called thin layer chromatography
(TLC). In TLC a very thin layer of an inert solid such as those mentioned as packing materials for
column chromatography is coated onto a glass or plastic plate. This layer of solid will serve as the
stationary phase in the chromatographic separation. The mobile phase will be a liquid, usually a
solution of organic liquids and/or water, as in column chromatography. A pencil is used to draw a
line about one centimeter from the bottom edge of the thin layer plate. Care must be taken not to
disturb the layer of solid on the plate. It is also important that the line be drawn with a pencil rather
than with an ink, because graphite from the pencil is a pure substance that is insoluble in most
common solvents. Most inks are mixtures of compounds that are soluble in organic liquids or in
water, so they could mask or interfere with the separation of the mixture of interest. A small drop
or spot of the mixture to be separated is placed on the line drawn on the thin layer plate. The plate
is carefully placed in a chromatography tank (often a beaker or flask) containing a small amount of
solvent. The solvent level should be lower than the level of the pencil line and sample spot. This
is very important because if the solvent covers the spot, it will dissolve the sample and wash it off
the plate. The TLC plate must be positioned so that it does not contact the walls of the tank as this
will alter the flow of the mobile phase. The tank is then covered tightly to prevent the evaporation
of the solvent and placed where it will not be bumped or jostled as that could change the position of
the plate inside. The solvent travels up the plate between the particles in the thin layer of solid by a
force called capillary action. Capillary action is the result of attractions the molecules in the liquid
feel for molecules on the surface of a solid. If solid surfaces are very close together, as they are in
the thin layer of particles on the plate, the liquid will fill the available spaces and thus travel slowly
up the plate. The solvent will carry the sample of mixture along the thin layer and the separation
will occur just as in column chromatography. The components more strongly attracted to the
stationary phase will remain near the bottom of the plate while those more attracted to the solvent
will be carried to the top. When the solvent nears the top of the plate, the plate is removed from the
tank. The highest level attained by the solvent is marked on the plate with a pencil. The TLC plate
with the separated mixture is called a chromatogram.
A technique very similar to TLC is paper chromatography. The major difference is that a
sheet or strip of paper is used instead of the thin layer plate. The stationary phase in paper
chromatography is not the paper, as one might guess, but rather a layer of water molecules
adsorbed onto the surface of the paper. Paper consists of molecules of a substance called cellulose
which is very attractive to water molecules. The mobile phase is again a liquid solution, usually
containing water and an organic liquid. In paper chromatography both the stationary and mobile
phases are liquids! Any type of paper can be used, but best results are obtained with special
chromatography paper.
Two other very important types of chromatography are gas - liquid chromatography (GLC
or GC) and high performance liquid chromatography (HPLC). These methods are used widely in
both research and commercial chemistry laboratories. They require the use of moderately to very
expensive equipment and instruments, but they produce results more rapidly and more accurately
than the traditional column, paper and thin layer chromatography methods. It is often possible to
determine the relative amounts of components in the mixture and the identity of components using
these techniques.
GC instruments contain a long metal column filled with chunks of an inert solid which
have been coated with a nonvolatile liquid. The liquid is the stationary phase. The mobile phase is
an inert gas, usually helium, which is pumped through the column. A tiny sample of the mixture
to be separated (1 mL or less) is injected into a heated block. The sample is vaporized and then
carried through the column by the helium. When the components exit the column, an electronic
detector measures both the presence and the relative amount of each one. From this data, one can
calculate the percent composition of the mixture. It is sometimes possible to identify the
components based on the amount of time that they spend in the column, the retention time. Under
identical conditions, a substance will always have the same characteristic retention time. Different
substances consist of different molecules which interact with the stationary phase differently, and
so have different retention times. If conditions in the column change, however, the retention times
will also change. One must be very cautious in attempting to identify substances based only on
retention time! (Retention time can also be measured with TLC and paper chromatography. For
these methods, one must determine what fraction of the total distance traveled by the solvent was
also traveled by each component of the mixture. This fraction is called the Rf value. Rf values are
discussed in more detail later in this experiment.) In order to identify the components of a mixture
with the greatest possible degree of confidence, a GC instrument can be connected to a mass
spectrometer (MS). The components of the mixture are analyzed by the mass spectrometer as they
exit the GC column. When connected to a computer, the GC/MS can report the identity and
relative amounts of each component of a complex mixture only minutes after a tiny sample has
been injected.
HPLC is similar to GC except that the mobile phase is a liquid, usually a solution of
organic liquids, which is pumped through the column under high pressure. The advantage of
HPLC over GC is that it can be used for mixtures that are difficult or impossible to vaporize.
EXPERIMENT 1: CLASSIFICATION AND SEPARATION
OF MATTER
EQUIPMENT: PART II: Chromatography
Use the space below to draw several pictures of the paper strip as the separation occurs. Be sure to
clearly label any colors you see.
What colors do you observe and in what order?
Rf =
so the Rf value for spot 1 is given as
Rf =
Determine the R value for each of the components of the mixture. Compare your values to
those obtained by other students in your class.f
EXPERIMENT 1: CLASSIFICATION AND SEPARATION
OF MATTER
Procedure B: Separations Using a Solvent Gradient
Obtain two 50 mL beakers. Fill one with deionized water and the other with methanol.
Draw 10 mL of methanol into a syringe (Be sure you've taken the cap off the syringe!). Connect
the syringe to the Sep Pak and run the methanol through the Sep Pak into a 250 mL beaker which
will be used for waste collection. Pour the waste methanol into the designated waste container.
Remove the Sep Pak from the syringe and draw 10 mL of water into the syringe. Re-connect the
Sep Pak to the syringe and run the water through the Sep Pak into the waste beaker. Describe your
observations of the methanol before and after passing it through the Sep Pak.
Disconnect the syringe from the Sep Pak and then draw 9 mL of deionized water and 1 mL of
methanol into the syringe. Invert the syringe a few times so that the solution is mixed well. Again,
connect the syringe to the Sep Pak and allow this 10 mL solution of deionized water and methanol to
run through. Let the first 5 mL run into the second vial and the last 5 mL run into the third vial.
Describe the color of the solution in the vials and the Sep Pak.
It is important to keep these vials in order so that you can see how the color changes.
Continue to follow the same procedure using 8 mL deionized water and 2 mL methanol;
7 mL deionized water and 3 mL methanol;
and 6 mL deionized water and 4 mL methanol.
Obtain a black Flair[TM] pen. File a deep groove in the barrel of the pen. Grasp the pen on
either side of the file mark and carefully snap the plastic barrel. Use forceps to remove the ink
cartridge. Do not touch the cartridge with your hands. Carefully break or cut the cartridge into
lengths of 1 to 2 cm. Share the pieces with others in your class.
Swirl a section cartridge in 50 mL of deionized water for a few seconds. Use the Sep Pak
to separate the components of this solution as you did above. Use the space below to describe
your procedure. Record your observations in a clear and organized manner.
Flair[TM] pen (Continued)
EXPERIMENT 1: CLASSIFICATION AND SEPARATION
OF MATTER
Students should work in pairs on this experiment.
In this experiment some properties of three substances will be studied. Based upon the observed
properties a scheme must be developed to separate the components of a mixture.
Before beginning the experiment several techniques which will be useful in completing the
separation of a mixture will be demonstrated by your instructor.
Use the space below to record important notes.
Section A:
Prepare a mixture that contains 0.1 g of sodium chloride, 20 mL of ethyl alcohol and 80
mL of deionized water in a 250 mL flask. Stir this mixture until the sodium chloride is dissolved.
Label the mixture "Test Solution" and stopper the flask.
Place 8 - 10 drops of the test solution onto a watch glass. Using a Bunsen burner (Figure
III) try to ignite it - be careful; if it can be ignited it will light quickly and produce a very hot flame.
What happens when you try to ignite the mixture?
Try reacting each of the components of the mixture with the silver nitrate solution. Clean
the watch glass and add 8 - 10 drops of deionized water, 1 drop of dilute (6 M) nitric acid and 1
drop of 0.1 M silver nitrate. Then clean the watch glass and add a small amount of solid sodium
chloride and 8 - 10 drops of deionized water, 1 drop of nitric acid and 1 drop of silver nitrate.
Finally, to a clean watch glass add 8 - 10 drops of ethanol, 1 drop of nitric acid and 1 drop of
silver nitrate. Describe what happens with each component in the space below.
What do these tests (Obs. #1 - #4) show you?
The receiving test tube should be placed in a 600 mL beaker half filled with water. This
test tube should not touch the bottom of the beaker.
Be sure the 2-hole stopper fits snugly into the sample test tube. Now you are ready to
begin distilling. If a hot plate or heating mantel is available it should be used rather than the
burner. You will have much more control of the rate of temperature change if a hot plate is used.
Begin heating the liquid.
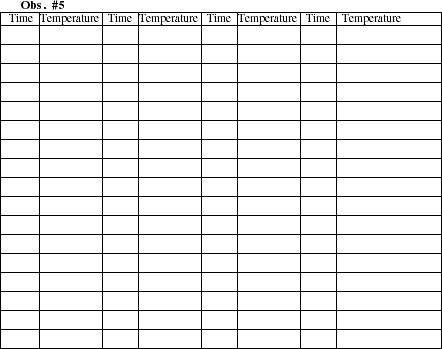
When the first drops of liquid begin to condense in the receiving test tube, read and record
the temperature in the table below. (Note: The thermometer is positioned so that it measures the
temperature of the vapor that enters the glass tube. This temperature is equal to the boiling point of
the vaporized substance.) Enter the time as 0 for this first reading. Continue recording the
temperature at 30 second intervals until the liquid in the sample test tube has boiled almost to
dryness. Heating the solution at a rate of no more than 2 degrees per minute is likely to yield good
results.
Use the sheet of graph paper to make a graph plotting the boiling temperature (y-axis) as a
function of time (x-axis). Draw a smooth curve connecting the data points. Describe the features
of the curve in space below.
What are the boiling points for pure ethanol and for pure water? (Note: Use the CRC
Handbook of Chemistry and Physics or any standard chemistry text.)
Prepare a table which better organizes the experimental observations. Describe a procedure
you could use to separate the mixture. Your description should include text, a flow chart , or
both.
(a) a dye. Several milliliters are available for testing.
(b) a handful of sand in a gallon of gasoline.
Note: Questions 5 and 6 relate to the distillation experiment. Students who
have not performed that experiment may need assistance to answer these
questions.
B

![]()
Obtain an unknown mixture of sand, sodium chloride, and calcium carbonate from your
instructor. Record the unknown number.
Unknown Number _______________
Mass of evaporating dish ...................... ______________ g
![]()
Add about 1 gram of the unknown to the evaporating dish and reweigh.
![]()
Mass of evaporating
dish + unknown................................ ______________ g
![]()
Mass of original sample........................ ______________ g
![]()
![]()
![]()
Identity of Component A _______________![]()
Weigh a piece of filter paper to use in the next step.
![]()
Mass of filter paper............................______________ g
![]()

![]()
![]()
![]()
Mass of Component A ........................ ______________![]()
Pour 5 mL of 6 M HCl (hydrochloric acid) into the funnel containing components B and C.
Upon addition of the 6 M HCl stir the solid gently with a clean glass stirring rod, being careful not
to tear the filter paper.
Add an additional 2 mL of dilute (6 M) HCl to be sure the reaction is complete. How can
you tell that the reaction is complete?
Expl. #3
![]()
If the water were evaporated from the solution above, what would be the identity of the
solid residue?
Identity of Solid Residue ______________
Identity of Component B ______________
![]()
Mass of filter paper +
Component B after drying ....................______________ g
![]()
Mass of Component B.........................______________ g![]()
Mass of Component C.........................______________ g
![]()
![]()
% of sand in original sample .................______________ %
% of NaCl in original sample.................______________ %
![]()
![]()
![]()
PART II: Chromatography
Introduction and Theory:
![]()
![]()
![]()
![]()
chromatography paper........about 12 inches ![]() scissors .........................1
scissors .........................1Flair[TM] pen ............................1 ![]() stopper for 250 mL flask .....1
stopper for 250 mL flask .....1flask, Erlenmeyer, 250 mL............1 ![]() pencil............................1
pencil............................1funnel ....................................1 ![]() ruler .............................1
ruler .............................1graduated cylinder, 10 mL ............1 
Obs. #1
![]()
What are the stationary phase and the mobile phase in this experiment?
Expl. #1
![]()
Why does the solvent move up the paper strip?
Expl. #2
![]()
![]()
Obs. #2
![]()
The colored dyes present in the ink move up the paper at different rates of speed,
depending on their attraction to the paper and solubility in the solvent. The spots usually move
more slowly than the solvent, so they cover less distance. (See the diagram below.) The fraction
of the distance traveled by each spot is dependent on the sample, the solvent, and the paper. It is
called the Rf value and is characteristic of the system. Rf values are calculated as shown:
![]()
![]()
![]()
When measuring the distance traveled by the spot you should use the center of the most
intensely colored part of the spot. Mark with a pencil the point at which you are making your
measurement.

![]()
![]()
Expl. #3
![]()
Staple your chromatogram to your paper in the space provided after it has dried.

![]()
![]()
EQUIPMENT:
beaker, 50 mL ..........................2
file........................................1
beaker, 250 mL.........................2
graduated cylinder, 50 or 100 mL....1
Flair Pen.................................1
Sep Pak..................................1
labels.................................... 10
syringe, disposable, 10 mL ...........1
forceps...................................1
vials ..................................... 10
![]()
PART II: Chromatography
Obs. #1
![]()
Why is it necessary to run the methanol and water through the Sep Pak before beginning
the experiment?
Expl. #1
![]()
Add a drop of yellow food coloring and a drop of blue food coloring to a clean 250 mL
beaker containing 100 mL of water. Stir the mixture. What color is produced?
Obs. #2
![]()
Label the vials with numbers 1 through 10. Using the syringe, obtain about 10 mL of the
colored solution and pass it through the Sep Pak, letting the liquid run into the first empty vial.
Describe the color of the solution in the vial and the Sep Pak.
Obs. #3

Obs. #4

Obs. #5

Obs. #6

Obs. #7

![]()
Clean your Sep Pak by drawing 10 mL portions of methanol into the syringe, attaching the
Sep Pak and running the liquid through the column into the waste beaker. Continue this until both
the column and the solvent are colorless. Clean the 10 vials by washing with deionized water.
![]()
![]()
![]()
What colors of dye are contained in the black Flair[TM] pen ink?
![]()
How do the results of this separation compare with the paper chromatography separation
performed on the same ink in Part II?
![]()
![]()
EQUIPMENT:
balance...................................1
rubber stopper,#4, 2 hole, slpit... 1
beaker, 600 mL.........................1
rubber stopper, to fit flask......... 1
Bunsen burner ..........................1
rubber tubing, 1 ft.................. 1
dropper ..................................1
rubber tubing, 2 ft ................. 2
flask, 250 mL...........................1
spatula ............................... 1
glass elbow for distillation ............1
test tube, 13 x 100 mm ............12
glass stirring rod........................1
test tube, 25 x 200 mm ............ 2
matches or lighter........................
thermometer, ºC .................... 1
graduated cylinder, 50 or 100 mL....1
utility clamps........................ 2
hot plate............................ 1(optional)
watch glass, 150 mm .............. 1
labels................................. several
weighing pan or paper ............. 1
ring.......................................1
wire gauze........................... 1
ring stand................................2
![]()
PART III: Distillation (optional) Notes
![]()
![]()

Obs. #1
![]()
Try igniting the components of the mixture. Clean the watch glass and add a small amount
of solid sodium chloride and try to ignite it; clean the watch glass and add 8 - 10 drops of ethanol
and try to ignite it (Note: Use caution because any flame produced will be difficult to see, yet easy
to feel); finally, clean the watch glass and add 8 - 10 drops of water and try to ignite it. Describe
what happens with each component in the space below.
Obs. #2
![]()
Now place about 5 drops of the test solution onto a clean watch glass. Add 1 drop
of 6 M nitric acid and 1 drop of 0.1 M silver nitrate solution. Describe what happens. Keep this
mixture for a future test (Obs. #5).
Obs. #3
![]()
![]()
Obs. #4
![]()
Which of the three components does the silver nitrate react with?
Expl. #1
![]()
Why was sodium chloride dissolved in water before adding the nitric acid and silver
nitrate?
Expl. #2
![]()
![]()
Expl. #3
![]()
Section B:
Set up an apparatus like the one shown in Figure IV. Poor technique can result in serious
cuts or punctures. If you are unsure how to proceed ask your instructor. Before inserting either
the thermometer or the glass elbow into the rubber stopper, wet the rubber stopper and the end of
the thermometer or glass elbow to be inserted with a glycerine/water solution. Wrap the remaining
part of the thermometer or glass elbow with a piece of paper towel and carefully insert the end into
the hole in the stopper. The thermometer should be inserted into the split hole of the stopper, while
the glass elbow should be inserted into the nonsplit hole. With a slight rotating movement, ease
the thermometer and glass elbow through the holes - DO NOT FORCE.
Adjust the end of thermometer and the end of the glass elbow to equal depths. To better
control the heating of the liquid sample arrange a wire gauze between the test tube and the Bunsen
burner. This will prevent the solution from heating too rapidly or too slowly. To prevent
superheating of the liquid in the distillation test tube, which could result in violent 'bumping', add
two or three boiling chips to the liquid at the beginning of the distillation. The boiling chips consist
of a porous material which act as a site for bubble formation. This will minimize superheating.
![]()

Expl. #4
![]()
![]()
Obs. #6
![]()
Considering the features of your graph and the boiling point of the liquids in the mixture
how could you collect pure or nearly pure samples of ethanol and water from the mixture?
Expl. #5
![]()
Now distill the remaining volume (about 60 mL) of the test solution. Collect fractions of
the distillate in 13 x 100 mm test tubes. A fraction is a sample of distillate collected over a known,
limited temperature range. Fractions of greatest interest will include pure (or nearly pure) samples
of each component of the mixture. Be sure to label the test tubes with the temperature range for
each fraction . Use the information from your graph to help decide when to shift from one test
tube to another (just move the rubber tubing into the next test tube).
Place a sample of each fraction in a clean watch glass. Test each of these fractions you
have collected for flammability. (Be careful when trying to ignite each sample.) Test a second
sample of each fraction for the presence of sodium chloride. Prepare a table which contains these
test results for each fraction collected. Identify the primary component of each fraction.
Obs. #7
![]()
Explain your observations with respect to the presence of sodium chloride. Where is the sodium
chloride?
Expl. #6
![]()
![]()

![]()
![]()
C is soluble in Y.
A and D are insoluble in X.
A is insoluble in Y
C is soluble in X.
A is magnetic.
B is insoluble in Y.
B is soluble in X.
B and C are not magnetic.
D is insoluble in Y.
D is not magnetic.
![]() Briefly describe how you would separate each of the following mixtures using procedures
performed in this experiment. Explain why you chose each method.
Briefly describe how you would separate each of the following mixtures using procedures
performed in this experiment. Explain why you chose each method.
![]()
![]()
![]()
(c) a mixture of sand and water (Note: The sand is very fine, and some tiny pieces are
floating in the water).
![]()
(d) one liter of a solution containing water and ethanol.
![]()
(e) one drop of mixed pigments used in the production of green paint.
![]() In the paper chromatography experiment what would be some of the possible results of
changing mobile phase?
In the paper chromatography experiment what would be some of the possible results of
changing mobile phase?
![]() Why is it important to use a pencil to mark lines on the chromatography paper?
Why is it important to use a pencil to mark lines on the chromatography paper?
![]()
![]()
![]() What happens to the boiling point of a solution during distillation? Explain.
What happens to the boiling point of a solution during distillation? Explain.
![]()
Contents
![]() Return to
Return to![]() Index of Experiments
Index of Experiments