 Go to Main Index
Go to Main Index Go to Main Index
Go to Main Index AP Chemistry by Satellite Lectureguide
Student Edition
Chemical Thermodynamics
Chapter 17
Objectives
Following your study of this chapter, you should be able to
![]()

![]()
![]()

1. State the first law of thermodynamics.
![]()
2a. Write the mathematical equation which relates the standard heat of reaction to the heats of
formation of the reactants and products of the chemical reaction.
![]()
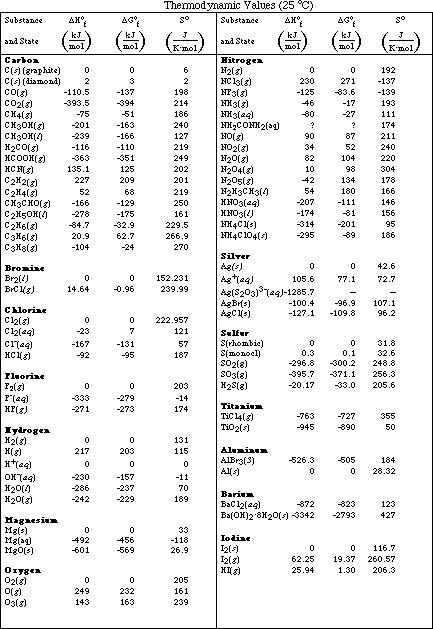
b. Calculate DHºreaction for the following chemical reactions. (Use the table of
thermodynamic values on page 19 - 24.)
![]()
![]()
Ans: -526.3 kJ
![]()
![]()
![]()
Ans: +58 kJ
![]()
![]()
![]()
![]()
Ans: 146 kJ
![]()
![]()
![]()
Ans: 890 kJ
![]()
3a. Define the terms exothermic and endothermic as they relate to chemical reactions.
![]()
b. Which of the reactions in Exercise #2b are exothermic and which are endothermic?

![]()
![]()
4. State the second law of thermodynamics.
![]()
5a. Define the term spontaneous and nonspontaneous as it relates to chemical change.
![]()
b. Based on our chemical intuition, which of the reactions in Exercise #2b are
spontaneous and which are nonspontaneous?

c. Is the enthalpy of a chemical reaction, the heat associated with a reaction at constant pressure, an accurate indicator of the spontaneity of a chemical reaction?
![]()
6. Define the term entropy. How is the sign of DS for a chemical reaction interpreted?
![]()
![]()
 7a. Re-state the second law of thermodynamics in terms of entropy.
7a. Re-state the second law of thermodynamics in terms of entropy.
![]()
b. Write a mathematical equation which relates the entropy change of the universe to the
entropy change of a system and its surrounding.
![]()
c. Predict whether the entropy of the system increases, remains constant or
decreases when the following processes occur. Explain your reasoning.
![]()
a) Ice melts at 0 ºC.
![]()
b) A precipitate forms in aqueous solution.
![]()
c) A solid dissolves in water.
![]()
d) A gas condenses to a liquid.
![]()
![]()
8. State the third law of thermodynamics.
![]()
9a. Write the mathematical equation which relates the standard entropy change in a chemical
reaction to the absolute entropy of the reactants and products.
![]()
b. Calculate DSºreaction for the following chemical reactions. (Use the table of
thermodynamic values on page 19 - 24.)
![]()
![]()
Ans: -72 J/K
![]()
![]()
![]()
Ans: +176 J/K
![]()
![]()
![]()
Ans: +592 J/K
![]()
![]()
iv) CO2(g) + 2H2O(l) D CH4(g) + 2O2(g)
![]()
Ans: +243 J/K
![]()
c. For which of the reactions in part b does the entropy increase, remain constant or
decrease?

d. Is the entropy of a chemical reaction an accurate indicator of the spontaneity of a chemical reaction?
![]()
![]()

![]()
10. Define the thermodynamic function called free energy. How is the sign of DGº for a
chemical reaction interpreted?
![]()
11. Write the mathematical equation which relates the standard free energy of a chemical
reaction to the enthalpy and entropy change of the reaction.
![]()
12a. Write the mathematical equation which relates the standard free energy of a chemical
reaction to the free energies of formation of the reactants and products.
![]()
![]()
b. Calculate DGºreaction for the following chemical reactions using both the equation in Exercise #11 and standard free energies of formation.
![]()
![]()
Ans: -505 kJ
![]()
![]()
![]()
Ans: +6 kJ
![]()
![]()
![]()
Ans: -32 kJ
![]()
![]()
![]()
Ans: +817 kJ
![]()
![]()
c. Which of the reactions in part b are spontaneous and which are nonspontaneous?

![]()
13a. Complete the blank cells in the table below. This table summarizes the effect of tem-
perature on the spontaneity of a chemical reaction. To complete the table, use the
information in the two left-hand columns to determine the entries of the remaining cells.
For example, in the first row the sign of DGºrxn (at 25 ºC) can be determined knowing
the sign of DHº and DSº for the reaction.
![]()

![]()
How do the magnitude of DHº and DSº change with temperature?
![]()
b. How does DGº change with increasing temperature for each of the following
reactions?
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
c. Calculate the temperature at which DGº is zero for the reaction
![]()
![]()
Ans: 345 ºC
![]()
![]()

14a. Write the mathematical equation which relates the free energy of a chemical reaction under standard conditions to the free energy of the reaction at nonstandard conditions.
![]()
b. DGº for the reaction,
![]()
![]()
was determined earlier. (Lesson #76, exercise 12b.)
![]()
i)Calculate K for the reaction at 25 ºC.
![]()
Ans: 8.89 x 10-2
![]()
ii) Calculate DG for the reaction if the partial pressure of NO2 is 0.1 atm and the
partial pressure of N2O4 is 1 atm.
![]()
Ans: -5.4 x 103 J
![]()
![]()
Problem Set #30
AP Chemistry by Satellite Name___________________________________
![]()
ALL work must be shown in all problems for full credit.
![]()
PS30.1. Using the table of thermodynamic values found on page 17 - 24, calculate the
DHºrxn (standard enthalpy change) for each of the following reactions:
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
PS30.2. For each of the following pairs, indicate which substance you would expect to possess the larger standard entropy:
![]()
a) 1 mol H2(g) at 298 K and 1 atm or 1 mol H2(g) at 298 K and at 10 atm.
![]()
b) 1 mol H2O(s) at 5 oC or 1 mol H2O(l) at 5 oC
![]()
c) 1 mol Br2(l) at 58.8 oC and 1 atm or 1 mol Br2(g) at 58.8 oC and 1 atm.
![]()
d) 1 mol KNO3(aq) at 30 oC or 1 mol KNO3(s) at 30 oC.
![]()
PS30.3. Predict whether the entropy change in the system is positive or negative for each of
the following processes:

![]()
PS30.4. For each reaction below, use the table of thermodynamic values on page 17 -
24 of the Lectureguide to determine the values of DHº and DSº.
![]()
![]()
![]()
![]()
PS30.4. (Continued)
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
PS30.4. (Continued)
![]()
![]()
PS30.5. a) Calculate DGº for each of the reactions in problem 30.4.
![]()
![]()
PS30.5. (Continued)
![]()
b) Which of the reactions in 30.4 are spontaneous at 298 K?
![]()
c) For each of the reactions listed in b), find the temperature above or below which
the reaction becomes nonspontaneous.
![]()
![]()
PS30.5. (Continued)
d) Which of the reactions in 30.4 are nonspontaneous at 298 K?
![]()
e) For each of the reactions listed in d), find the temperature above or below which
the reaction becomes spontaneous.
![]()
PS30.6. Consider the reaction
![]()
![]()
a) The concentration equilibrium constant, Kc, for this reaction is 1.12 x 10-3 at
750 K. Would you expect this value to increase or decrease at 300 K? Explain
your answer using words, no calculations yet!
![]()
b) Calculate DHº and DSº for the reaction from the table of thermodynamic values in
your text or from the table on page 17 - 24 of the Lectureguide.
![]()
![]()
PS30.6.(Continued)
![]()
c) Using the values obtained in b), calculate DGº for the reaction at 300 K and at 750
K.
![]()
d) Calculate Kc for the reaction at 300 K. Compare your value with your prediction in
a).
![]()
![]()
PS30.7. a) If 4.00 moles each of Cl2, H2O and O2 are placed into a 2.00 L vessel at 750 K, what will be the equilibrium concentration of HCl? (See PS30.6b.)
![]()
b) If the experiment described in a) is repeated at 300 K, what will the concentration of
HCl be?
![]()
PS30.8. Under what conditions do enthalpy, entropy and free energy take on values of zero?
![]()
![]()
PS30.9. a) Given Kb for ammonia is 1.8 x 10-5 at 298 K, calculate DGº for the reaction
![]()
![]()
![]()
b) What is the value of DG at equilibrium?
![]()
c) What is the value of DG when the [NH3] = 0.100 M, [NH4+] = 0.100 M and [OH-]
= 0.0500 M?
![]()
PS30.10. The enthalpy of combustion, DHºcomb, for oxalic acid, C2H2O4(s), is
![]()
![]()

a) Write the balanced chemical equation which describes the combustion of one mole of oxalic acid.
![]()
![]()
PS30.10. (Continued)
b) Write the balanced chemical equation which describes the standard formation of oxalic acid.
![]()
c) Using the information given above and the equations in a) and b), calculate DHºf
for oxalic acid.
![]()
d) Calculate DSºf for oxalic acid and DSºrxn for the combustion of one mole of
oxalic acid.
![]()
e) Calculate DGºf for oxalic acid and DGºrxn for the combustion of one mole of
oxalic acid.
![]()
f) Is the formation of oxalic acid from its elements spontaneous? Is the combustion
of oxalic acid at 25 ºC spontaneous?
![]()
![]()
![]()
![]()