 Go to Main Index
Go to Main Index Go to Main Index
Go to Main Index AP Chemistry by Satellite Lectureguide
Student Edition
Chemical Kinetics
Chapter 13
Objectives
Following your study of this chapter, you should be able to:
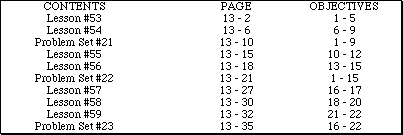
![]()
![]()

1. List four factors which affect the rate of a chemical reaction. For each provide a brief statement describing how it affects the speed of a chemical reaction.
![]()
2. Define the term chemical kinetics.
![]()
![]()
3. Define the term reaction rate.
![]()
For the following chemical reaction
![]()
![]()
write the rate equation in terms of the change in concentration of N2O5 with time,
D[NO2] with time and D[O2] with time.
![]()
4. Using the plot below, define the terms average rate, instantaneous rate and initial
rate.
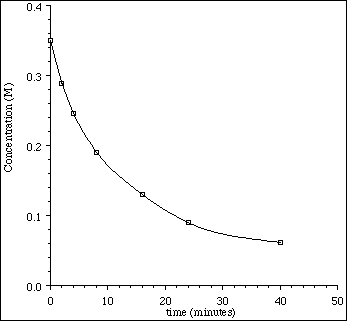
![]()
![]()
5a. Given the following data
![]()
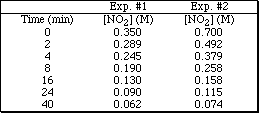
for the reaction 2NO2(g)  2NO(g) + O2(g)
2NO(g) + O2(g)
Plot the data for Exp. #1 and determine the average rate of the reaction between 8 and 24 min., the instantaneous rate of the reaction at 8 minutes and the initial rate of the reaction.

![]()
![]()
b.Plot the data for Exp. #2 and determine the average rate of the reaction between 8 and 24 minutes, the instantaneous rate of the reaction at 8 minutes and the initial rate of the reaction.

c. By what factor did the initial concentration change in going from Exp #1 to Exp #2?
![]()
d. By what factor did the initial rate change in going from Exp #1 to Exp #2?
![]()
e. Write an equation which describes how the initial rate of the reaction depends on
the initial concentration.
![]()
![]()

6. Define the terms; rate equation and rate law for a chemical reaction.
![]()
7. Write the general rate law for the following reaction;
![]()
![]()
Identify the rate constant in the rate law. What are the exponents in the rate law
called?
![]()
![]()
8. What experimental data is needed to determine the order of a chemical reaction?
![]()
a. Consider the reaction
2 NO(g) + 2 H 2(g)
 N2(g) + 2 H2O(g)
N2(g) + 2 H2O(g)
and the following initial rate data.
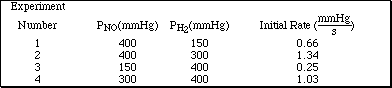 i)
i)
Determine the reaction order for NO and H2.
![]()
Ans: H2 is 1st order and NO is 2nd order
ii) Determine the overall order of the reaction.
![]()
iii) Write the specific rate law for the reaction.
![]()
Ans: rate = k(PNO) 2(PH2) 1
![]()
Ans: k = 2.7 x 10-8 mmHg-2·sec-1
![]()
![]()
8b. The following initial rate data were collected for the reaction
![]()
at 100 ºC. (Problems: BL 15.15 - 15.16)
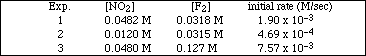
![]()
i) Determine the reaction order for NO2 and F2.
![]()
Ans: F2 is 1st order and NO2 is 1st order
![]()
ii) Determine the overall order of the reaction.
![]()
iii) Write the specific rate law for the reaction.
![]()
Ans: rate = k[NO2] 1[F2] 1
![]()
iv) Determine the rate constant for the reaction (include units).
![]()
Ans: k = 1.24 M-1.sec-1
![]()
![]()
c. For the reaction
![]()
and the following initial rate data.
![]()

![]()
i) Determine the reaction order for A, B and C.
![]()
ii) Determine the overall order of the reaction.
![]()
iii) Write the specific rate law for the reaction.
![]()
Ans: rate = k[A]2[Y]1[C]1/2
![]()
iv) Determine the rate constant for the reaction (include units).
![]()
Ans: k = 998 M-2.5.sec-1
![]()
![]()
Problem Set #21
AP Chemistry by Satellite Name___________________________________
![]()
ALL work must be shown in all problems for full credit.
PS21.1. The following data was collected for the reaction
![]()
![]()
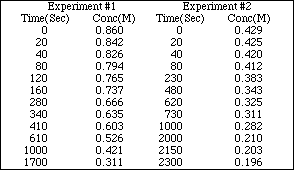
a) Plot the data for Exp. #1 and graphically estimate
![]()
![]()

![]()
![]()
PS21.1. (Continued)
i) the initial rate
![]()
ii) the instantaneous rate at 100 sec? 750 sec? 1350 sec?
![]()
iii) the time it takes for half of the HI to react
![]()
b) Repeat a) for Exp #2
i) the initial rate
![]()
ii) the instantaneous rate at 100 sec? 750 sec? 1350 sec?
![]()
iii) the time it takes for half of the HI to react
![]()
c) By what factor did the initial concentration change in going from Exp #1 to Exp #2?
![]()
d) By what factor did the initial rate change in going from Exp #1 to Exp #2?
![]()
![]()
PS21.1. (Continued)
e) What is the order of the reaction with respect to HI?
![]()
f) How did the half-life change for the two experiments?
![]()
g) Determine the rate constant for the reaction including units.
![]()
h) What would the initial rate be if the concentration of HI is 0.654 M? 1.25 x 10-2 M?
![]()
![]()
PS21.2. The following initial rate data were collected for the reaction
![]()
at 25 ºC.
![]()
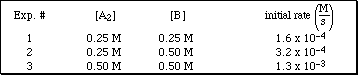
a) Determine the reaction order for A2 and B.
![]()
b) Determine the overall order of the reaction.
![]()
c) Write the specific rate law for the reaction.
![]()
d) Determine the rate constant for the reaction (include units).
![]()
![]()
PS21.3. The following initial rate data were collected for the reaction
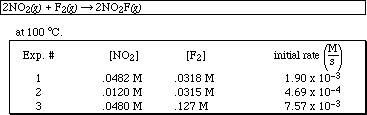
![]()
a) Determine the reaction order for NO2 and F2.
![]()
b) Determine the overall order of the reaction.
![]()
c) Write the specific rate law for the reaction.
![]()
d) Determine the rate constant for the reaction (include units).
![]()
![]()

10a. Write the stepwise derivation of the general mathematical equation which relates concentration to time for a simple first order reaction.
![]()
b. The decomposition of H
constant of 0.0410 min-1 at a particular temperature.2O2 to H2O and O2 follows first order kinetics with a rate
![]()
![]()
Calculate the [H2O2] after 10 mins if [H2O2]0 is 0.200 M.
![]()
Ans: 0.133 M
![]()
![]()
c. The decomposition of N2O5 to O2 and NO2 follows first order kinetics. If a sample at 25 ºC with the initial concentration of N2O5 of 1.25 x 10-3 M falls to 1.02 x 10-3 M in 100. minutes, calculate the rate constant for the reaction.
![]()
Ans: 2.03 x 10-3 min-1
![]()
11a. Derive a mathematical equation for the half-life for a reaction which follows
simple first order kinetics.
![]()
b. In Problem 10b, how long would it take for half of the H2O2 to decompose?
![]()
Ans:16.9 min
![]()
![]()
12a. Show how a plot of ln[concentration] versus time can provide the rate constant for a reaction which follows simple first order kinetics.
![]()
b. Using the following data, establish that the decomposition of N2O5 according to the
reaction,
![]()
follows first order kinetics. Determine the rate constant for the reaction.
![]()

![]()
![]()

13a. Write the stepwise derivation of the general mathematical equation which relates concentration to time for a simple second order reaction.
![]()
b. The decomposition of NOCl(g)
![]()
![]()
is a second order reaction with a rate constant of 0.0480 M-1·sec-1 at 200 ºC. In an
experiment at 200 ºC, the initial concentration of NOCl was 0.400 M. What is the
concentration of NOCl after 15.0 min have elapsed?
![]()
Ans: 0.0218 M
![]()
![]()
c. How many minutes will it take for the concentration of NOCl(g) to drop to 0.150 M?
![]()
Ans: 86.8 s
14a. Derive a mathematical equation for the half-life for a reaction which follows simple second order kinetics.
![]()
b. The initial concentration of NOCl, described in 13b above, is 0.400 M. Calculate the
half-life for the decomposition reaction.
![]()
Ans: 52.1 s
![]()
![]()
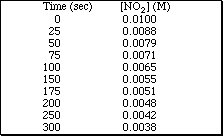
15a. Show how a plot of ln [concentration] versus time can provide the rate constant for a reaction which follows simple second order kinetics.
![]()
b. Using the following data establish that the decomposition of NO 2 according to the
reaction,
![]()
following second order kinetics. Determine the rate constant for the reaction.
![]()
![]()
![]()

![]()
![]()
Problem Set #22
AP Chemistry by Satellite Name___________________________________
![]()
ALL work must be shown in all problems for full credit.
PS22.1. The reaction
 N2O4(g)
N2O4(g)
follows simple second order kinetics. If the [NO2]0 is 0.156 M,
a) calculate the rate constant for the reaction if it takes 1.00 x 10-3 s for the concentration of NO2 to fall to 0.147 M.
![]()
b) calculate the half-life for the reaction. (When the [NO2]0 = 0.156 M.)
![]()
c) how long will it take for the [NO2] to fall to 5.00 x 10-2 M?
![]()
d) what is the [NO2] after 1.00 s? (When [NO2]0 = 0.156 M.)
![]()
PS22.2. The reaction
![]()
follows simple first order kinetics with a half-life of 12.4 s.
a) Calculate the rate constant for the reaction.
![]()
![]()
PS22.2. (Continued)
![]()
b) How long will it take for the [H2O2] to fall from 0.300 M to 0.0452 M?
![]()
c) What is the [H2O2] after 30 minutes if [H2O2]0 = 1.25 M?
![]()
d) How long will it take for the [H2O2] to decrease by a factor of 6?
![]()
PS22.3. C4H8 decomposes according to the following equation;
![]()
![]()
the rate constant for the decomposition is 6.07 x 10-10 sec-1 at 25 ºC.
![]()
a) What is the order of the reaction?
![]()
b) How long would it take for 1.00 % of a sample of C4H8 to decompose at 25 ºC
and 1 atm?
![]()
![]()
PS22.3b. (Continued)
![]()
c) What is the half-life of the reaction?
![]()
d) How long would it take for 1.00 % of a sample of C 4H8 to decompose at 25 ºC
and 10 atm?
![]()
![]()
PS22.4. The second-order decomposition of nitrous oxide, N2O, has a half-life of 75.0 min at 900 K when the initial concentration of N2O is 2.00 x 10-2 M.
![]()
a) What is the concentration of nitrous oxide after 150 minutes?
![]()
b) How long will it take for 40.0 % of the sample to decompose?
![]()
![]()
PS22.5. The first-order rate constant for the reaction
![]()
![]()
is 4.00 x 10-4 sec-1 at 573 K.
![]()
a) What will be the concentration of CH3N2CH3 after 600 seconds, given that the
initial concentration is 1.03 x 10-2 M?
![]()
b) What is the half-life of the reaction for this initial concentration?
![]()
![]()
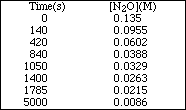
PS22.6. In the reaction
![]()
![]()
the [N2O] was followed with time and the data shown below was obtained.
![]()
![]()
Determine the order of the reaction and its half-life.
![]()

![]()
![]()

16a. The following rate data was obtained at different temperatures for the reaction
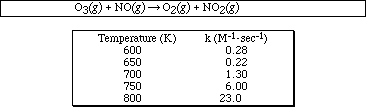
Sketch the plot of ln k (y-axis) versus ( 1/temperature ) (x-axis).
![]()
![]()

![]()
![]()
b. Define the term activation energy.
![]()
17a. Write the Arrhenius equation and define each term.
![]()
b. At 300 ºC the rate constant for the reaction
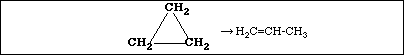
![]()
is 2.41 x 10-10 sec-1. At 400 ºC the rate constant is 1.16 x 10-6 sec-1. Calculate the activation
energy for the reaction.
![]()
Ans: Ea = 272 kJ/mol
![]()
![]()
c. Estimate the rate of the rearrangement reaction at 800 ºC.
![]()
Ans: k = 84.1 s-1
![]()
d. If the activation energy for the decomposition of N2O5 is 1.0 x 102 kJ/mol,
calculate the temperature change necessary to double the rate at room temperature.
![]()
Ans: DT = 5 K
![]()
![]()

18. Sketch the energy profile diagram for the exothermic reaction
![]()
and label the important features, including reactants, products, activated
complex, the energy of activation and the enthalpy of the reaction.
![]()
![]()
19a. Define the terms reaction mechanism and rate determining step.
![]()
b. List several characteristics of a reasonable mechanism for a chemical reaction.
![]()

![]()
![]()

21a. Define the terms unimolecular, bimolecular and termolecular.
![]()
b. Suggest a mechanism for the reaction
![]()
![]()
if the experimental rate law is rate = k[CH3NC]1.
![]()
c. Suggest a mechanism for the reaction
![]()
![]()
if the experimental rate law is rate = k[CH3Br]1[OH-]1.
![]()
![]()
d. Suggest a mechanism for the reaction
![]()
![]()
if the experimental rate law is rate = k[NO2]2.
![]()
e. Suggest a two step mechanism for the reaction
![]()
![]()
if the experimental rate law is rate = k[NO2Cl]1.
![]()
22. Define the terms reaction intermediate and catalyst.
![]()
![]()
Problem Set #23
AP Chemistry by Satellite Name___________________________________
![]()
ALL work must be shown in all problems for full credit.
![]()
PS23.1. The activation energy for the decomposition of N2O5 is 102 (kJ/mol). The rate
constant for the reaction at 45 ºC is 5.00 x 10-4 M-1·sec-1. What is the value
of the rate constant at 65 ºC?
![]()
PS23.2. Using the data in PS23.1, calculate the temperature at which the rate constant is
3.00 x 10-6 M-1·sec-1.
![]()
![]()
PS23.3. Using the data in PS23.1, calculate the rate constant at 0 ºC.
![]()
PS23.4. A chemist was able to determine that the rate of a particular reaction at 100 ºC
was four times faster than at 30 ºC. Calculate the approximate energy of
activation for such a reaction.
![]()
![]()
PS23.5. Data for the reaction
![]()
was collected and is shown below. Plot the data and determine the activation energy for the reaction.
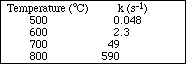

![]()
![]()
PS23.6. Consider the simple reaction,
![]()
Determine what the order of the reaction must be for each statement to be true. a) The initial concentration of A is doubled and the initial rate increase by a factor of four.
![]()
b) The half-life for the disappearance of A is inversely proportional to the initial
concentration of A.
![]()
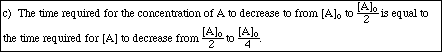
![]()
PS23.7. Given the following reaction mechanism
![]()
What is the overall reaction? Write the rate law for the reaction.
![]()
![]()
PS23.8. If the reaction
![]()
occurred via a one step mechanism, draw a picture of activated complex.
Discuss how likely a one-step mechanism would be for this reaction.
![]()
PS23.9. Suggest a two step mechanism for the reaction
![]()
![]()
if the experimental rate law is rate = k[NO][Cl2].
![]()
PS23.10. The suggested mechanism for the reaction between peroxide and iodide ion is,
![]()
Identify a specie(s), if any, which is acting as a catalyst and a specie(s) which is acting as an intermediate.
![]()
![]()