Go to Main Index
Go to Main Index Go to Main Index
Go to Main Index
Name ________________________
AP Chemistry By Satellite
John I. Gelder
Exam III
April 9, 1992
![]()
INSTRUCTIONS:
1. This examination consists of a total of 12 different pages. The last 4 pages include important mathematical equations and constants, a solubility table, a periodic table, and tables of dissociation constants, solubility constants and thermodynamic values. All work should be done in this booklet. You may carefully remove the last 4 pages of the examination.
2. PRINT your name, high school, teaching partner's name and today's date now in the space at the top of this sheet. DO NOT SEPARATE THE PAGES.
3. Answer all questions that you can and whenever called for show your work clearly. Your method of solving problems should pattern the approach used in lecture. You do not have to show your work for the multiple choice or short answer questions. Clearly state any assumptions used when solving equilibrium problems.
4. No credit will be awarded if your work is not shown in problem 2 - 8. Please circle your final answer!
5. Point values are shown next to the problem number.
6. Budget your time for each of the questions. Some problems may have a low point value yet be very challenging. If you do not recognize the solution to a question quickly, skip it, and return to the question after completing the easier problems.
7. Look through the exam before beginning; plan your work; then begin.
![]()
8. Relax and do well.
![]()

APCBS Exam III PAGE 2
![]()
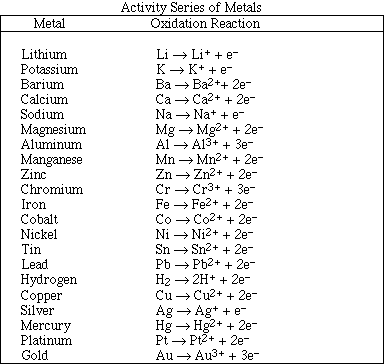
(12) 1. Complete and balance four of the following reactions. Identify the phase of each product as either
(g)as, (l)iquid, (s)olid or (aq)ueous. Products which are soluble ionic compounds must be written as
ions. If no reaction occurs, write NR.

![]()
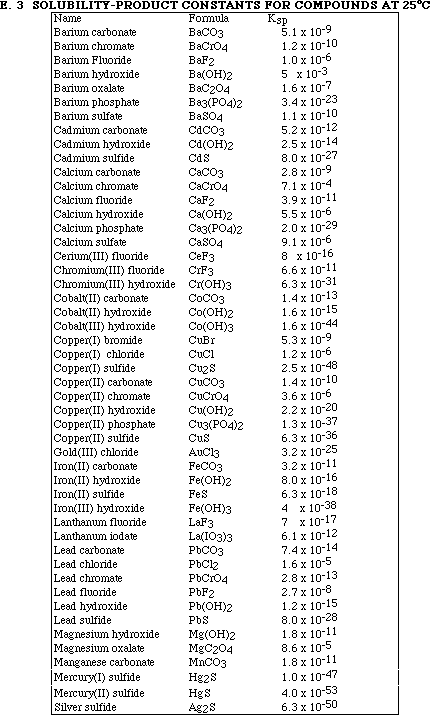
(4) 2. Calculate the magnitude of the equilibrium constant for one of the reactions in Problem #1.

![]()
Note:
Ksp for d) and e) were not included on the extra information page, but the information
to calculate values for a), b) and c) was included.
![]()

APCBS Exam III PAGE 3
![]()
(10) 3. Answer ONE of the following questions.
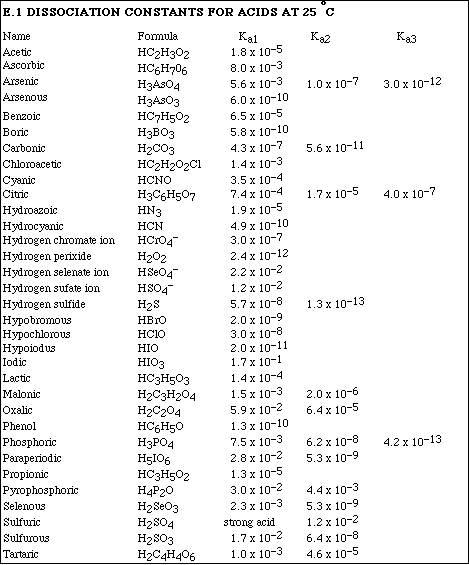
a) Calculate the pH of a solution which is 0.345 M NaBrO.

![]()
b) Calculate the pH of a solution which is 0.345 M NH4Cl.
![]()

APCBS Exam III PAGE 4
![]()
(10) 4. Answer ONE of the following questions;
![]()
a) Calculate the pH of a solution prepared by mixing 35.0 mL of 0.200 M HCl and 50.0 mL of 0.175 M
NH3.
![]()

b) Calculate the pH of a solution prepared by mixing 36.0 mL of 0.350 M HNO 3 and 18.0 mL of 0.600 M KOH.

APCBS Exam III PAGE 5
![]()
(20)5a. Calculate the pH of a solution which is 0.250 M HC3H5O2 (propionic acid) and 0.350 M KC3H5O2.

b) Calculate the pH after adding 0.025 moles of HCl to 500 mL of the buffer in Problem #5a.

![]()
+ Either moles or mol/L can be used at this point. If mol/L are used the value for both
[HC3H5O2] and [C3H5O2-] is 0.300 M.
APCBS Exam III PAGE 6
![]()
(8) 6. Calculate the solubility, in mol/L, for ONE of the following compounds in water.
![]()
a) LaF3
![]()

![]()
b) CrF3
![]()

![]()
(10) 7. Calculate the solubility, in mol/L, for the same compound in Problem 6 if it is dissolved in 0.500 M
KF.

APCBS Exam III PAGE 7
![]()

APCBS Exam III PAGE 8
![]()
Multiple Choice: (14 points)
Print the letter (A, B, C, D, E) which corresponds to the answer selected.
9. A ![]() 10. E
10. E ![]() 11. D
11. D ![]() 12. A
12. A ![]() 13. C
13. C
14. D ![]() 15. E
15. E
ONLY THE ANSWERS IN THE AREA ABOVE WILL BE GRADED. Select the most correct answer for each question. Each question is worth 2 points.
9. Which of the following compounds has the greatest solubility in mol/L?
A. CuCl (Ksp = 1.2 x 10-6)
B. CaCO3 (Ksp = 2.8 x 10-9)
C. BaC2O4 (Ksp = 1.6 x 10-7)
D. HgS (Ksp = 4.0 x 10-53)
E. FeS (Ksp = 6.3 x 10-18)
10. Which of the following is the weakest acid?
A. HC7H5O2(aq)
B. HCN(aq)
C. HONH3+(aq)
D. HC2H3O2(aq)
E. CH3N H3+(aq)
11. For which of the following reactions is DSº positive at 25 ºC?

12. Which of the following mixtures would produce a precipitate of PbCrO4?
A. 10.0 mL of 1.0 x 10-6 M Pb(NO3) 2 and 10.0 mL of 1.0 x 10-5 M K2CrO4 B. 100.0 mL of 1.0 x 10-6 M Pb(NO3)2 and 100.0 mL of 1.0 x 10-7 M K2CrO4 C. 25.0 mL of 1.0 x 10-7 M Pb(NO3)2 and 50.0 mL of 1.0 x 10-6 M K2CrO4 D. 50.0 mL of 1.0 x 10-6 M Pb(NO3)2 and 100.0 mL of 1.0 x 10-7 M K2CrO4 E. 33.0 mL of 1.0 x 10-7 M Pb(NO3)2 and 66.0 mL of 1.0 x 10-6 M K2CrO4
APCBS Exam III PAGE 9
13. The magnitude of the equilibrium constant for the following reaction is;
![]()
A) 5.6 x 1018
B) 1.0 x 1014
C) 1.8 x 109
D) 1.0 x 107
E) 1.8 x 105
14. If a titration is carried out with the following chemicals, which would have the highest equivalence point pH?
A. 0.100 M HNO3 and 0.100 M NaOH
B. 0.100 M HCl and 0.100 M NH3
C. 0.100 M H2SO4 and 0.100 M KOH
D. 0.100 M HC2H3O2 and 0.100 M NaOH
E. 0.100 M HBr and 0.100 M KOH
15. To make a buffer using HC2H3O2 and C2H3O2- in which the desired pH is 5.00, the [HC2H3O2] and [C2H3O2-] should be,
A. [HC2H3O2] = 1.00 M and [C2H3O2-] = 1.00 M
B. [HC2H3O2] = 0.100 M and [C2H3O2-] = 0.100 M
C. [HC2H3O2] = 0.250 M and [C2H3O2-] = 0.100 M
D. [HC2H3O2] = 0.750 M and [C2H3O2-] = 0.237 M
E. [HC2H3O2] = 0.139 M and [C2H3O2-] = 0.250 M