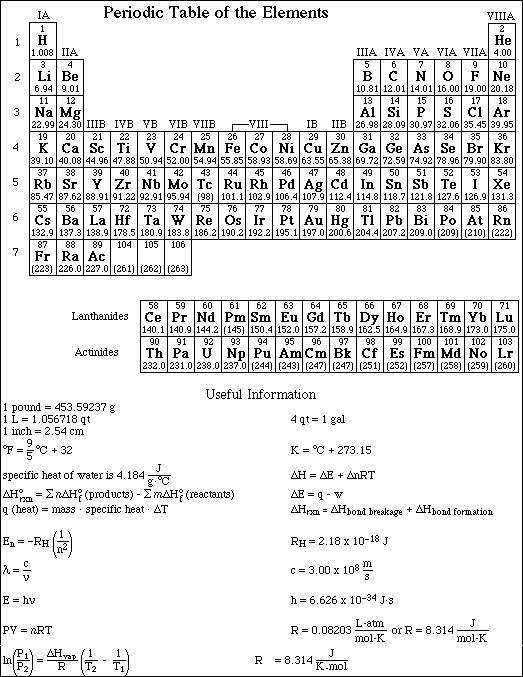Go to Main Index
Go to Main Index Go to Main Index
Go to Main Index Name ________________________
AP Chemistry By Satellite
Exam Final
John I. Gelder
December 19, 1990
INSTRUCTIONS:
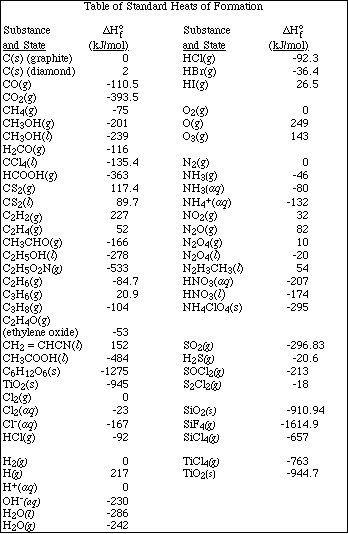
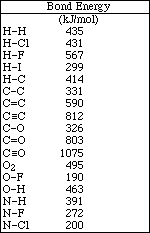
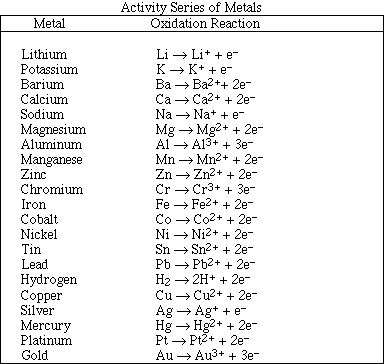
1. This examination consists of a total of 12 different pages. Page 2 - 6 contain all the multiple choice questions. The short answer questions are on page 7. The last 5 pages includes a periodic table, useful mathematical equations and constants, a table of standard heats of formation, an activity series and a table of bond energies. All work should be done in this booklet.
2. PRINT your name, high school and teaching partner's name now in the space at the top of this sheet. DO NOT SEPARATE THESE PAGES.
3. Questions 1 - 30 are multiple choice. Questions 31 and 32 are short answer. You do not have to show your work for the multiple choice or short answer questions.
5. Each multiple choice question is worth 3 points. Point values are shown next to the short answer questions.
6. Budget your time for each of the questions. Some questions will require less time to complete compared to others. If you do not recognize the solution to a question quickly, skip it, and return to the question after completing the easier problems.
7. Look through the exam before beginning; plan your work; then begin.
![]()
8. Relax and do well.
![]()

APCBS Exam VI PAGE 2
![]()
Multiple Choice:
Page 8 of this examination contains an answer sheet for the multiple choice questions. You may remove this sheet along with the 4 pages of useful information. Turn in the both page 7 and 8 for grading.
Questions 1 - 3
A) argon
B) sodium
C) oxygen
D) helium
E) carbon
1. The element which is a soft silvery metal.
2. Forms a covalent (network) solid.
3. A dense, colorless gas.
4. The bulb labeled A has a volume of 300 ml and contains a colorless gas at 500 mm Hg, while bulb B has a volume of 400 mL and contains a colorless gas at 400 mm Hg. If the valve separating the two bulbs is opened and the gases allowed to mix, calculate the final pressure of the mixture. Assume the temperature remains constant and the volume of the valve tube is negligible compared to the total volume.

![]()
A) 300 mm Hg
B) 442 mm Hg
C) 457 mm Hg
D) 900 mm Hg
E) 908 mm Hg
5. An element, X, with the electron configuration
1s22s22p63s23p3
will most likely form one of the following compounds.
A) NaX
B) C2X2
C) XO-
D) XCl3
E) AlX3
APCBS Exam VI PAGE 3
![]()
6. What is the wavelength of a photon light emitted when the electron in a hydrogen atom falls from n = 4
level to the n = 1 level?
A) 85 nm
B) 97 nm
C) 123 nm
D) 246 nm
E) 434 nm
7. Which of the following sets of quantum numbers is not allowed for an electron in a hydrogen atom?

8. How much heat is required to raise the temperature of 30.0 g of silver from 18.2 ºC to 35.6 ºC? The specific heat of silver is 0.226 J/g·ºC.
(A) 123 J
(B) 118 J
(C) 241 J
(D) 2.31 kJ
(E) 1.08 kJ
9. Which of the following statements is false?
(A) The reaction vessel cools when an endothermic reaction occurs.
(B) An exothermic reaction is characterized by a negative value of DH.
(C) Heat is evolved when an exothermic reaction occurs.
(D) Heat is added to the system in an endothermic reaction.
(E) An endothermic reaction causes the surroundings to absorb heat.
10. Calculate the amount of heat evolved in the complete oxidation of 8.17 g of Al at 25 ºC and 1 atm pressure. DHºf for Al2O3 is -1676 kJ/mol .
(A) 101 kJ
(B) 127 kJ
(C) 203 kJ
(D) 237 kJ
(E) 254 kJ
APCBS Exam VI PAGE 4
11. ![]() CCl4, CO2, PCl3, PCl5, SF6
CCl4, CO2, PCl3, PCl5, SF6
Which of the following geometries does NOT describe any of the molecules above?
A) octahedral
B) linear
C) trigonal planar
D) tetrahedral
E) trigonal bipyramidal
![]()
12. The relative atomic mass of one of two naturally occurring isotopes of chlorine is 34.969 u. The
percentage abundance of this isotope, 35Cl, is 75.53%. What is the relative atomic mass of the other
naturally occurring isotope of chlorine?
A) 35.00 u
B) 35.40 u
C) 36.00 u
D) 36.93 u
E) There is not enough information to answer the question.
![]()
13. How many hydrogen atoms are contained in a sample of (NH4)2CO3 with a mass of 3.188 x 10-22 g?
A) 2
B) 4
C) 8
D) 16
E) 32
![]()
14. A 1.622 g sample of a chloride salt of an unknown element, X, with the formula XCl3 is analyzed and
found to contain 0.03000 moles of Cl-. What is the symbol of the unknown element, X?
A) Al
B) In
C) Sc
D) Ga
E) Fe
15. Given the reaction,
![]()
![]()
If 4.16 g of KI, 2.08 g of KMnO4, 2.08 g H2O and 2.08 f of KOH are mixed together, which substance
is the limiting reagent? (Molar masses:
KI = 166 g;
KMnO4 = 197 g;
H2O = 18.0 g; KOH = 56.1 g)
![]()
A) KI
B) KMnO4
C) H2O
D) KOH
E) not enough information
APCBS Exam VI PAGE 5
![]()
16. If 34.5 g of C4H10 are combusted in excess oxygen, how many moles of H2O are formed?
![]()
A) 0.248 mol
B) 0.595 mol
C) 1.49 mol
D) 2.97 mol
E) not enough information
17. Which of the following do not appear to be in the correct order increasing atomic radius?
A) Mg2+ < Ca2+ < Ba2+
B) Na+ < F- < N3-
C) F- < Br- < I-
D) Cu < Ag < Au
E) Cr < Fe < Ni
![]()
18. The molecular ion XF2- has three pairs of nonbonding electrons around the central atom. The is the
bond angle around X will be closest to;
A) 180º
B) 120º
C) 109º
D) 107º
E) 90º
19. Which of the following statements is true?
![]()
A) The average kinetic energy of a sample of gas particles is inversely proportional to temperature.
B) The rate of effusion of SO2 is half as large as the rate of effusion of CH4.
C) The root mean velocity of an oxygen molecule is higher compared to a CH4 molecule.
D) The rate of effusion of CH4 is half as large as the rate of effusion of SO2.
E) The average kinetic energy of CH4 molecules is higher compared to oxygen molecules at the same
temperature.
![]()
20. If a liter of CO2 is compared to a liter of H2, both at 25ºC and one atmosphere pressure, then:
A) the CO2 and H2 molecules have the same average speed
B) there are more H2 molecules than CO2 molecules
C) the average kinetic energy of the CO2 molecules is greater than that of the H2 molecules
D) the CO2 molecules are on the average moving more slowly than the H2O molecules
E) the H2 molecules experience greater intermolecular attractive forces
APCBS Exam VI PAGE 6
![]()
21. Which of the following would be properly classified as a set of covalent molecules?
A) NaCl, Cl2, O2
B) CO2, HCN, O2
C) AgCl, ScF3, P4
D) CO2, NH4Cl, C2H6
E) BeH2, F2, AlI3
![]()
A) 18 protons, 18 neutrons and 18 electrons
B) 8 protons, 10 neutrons and 8 electrons
C) 8 protons, 10 neutrons and 10 electrons
D) 8 protons, 8 neutrons and 6 electrons
E) 8 protons, 10 neutrons and 6 electrons
23. Analysis of a hydrocarbon showed it contained 14.4% hydrogen and 85.6% carbon by weight. What is its empirical formula?
A) CH
B) CH2
C) CH3
D) C2H3
E) C2H5
24. Which of the following elements has the highest first ionization energy?
A) S
B) Ba
C) Si
D) O
E) B
25. The ability of an atom to compete for electrons with another atom to which it is bonded describes:
A) ionization potential
B) electronegativity
C) electron affinity
D) magnetism
E) Trouton's rule
26. Which of the following sets does not contains a polar molecule?
A)
H2S, CO, CO2
B)
BF3, ClF3, O2
C)
CO2, HCCH, XeF4
D)
H2S, HCN, CO2
E)
NH3, PF3, OCCl2
APCBS Exam VI PAGE 7
![]()
27. 9.33 grams of copper metal was allowed to react with an excess of chlorine, and it was found that 14.6
grams of a compound of copper and chlorine were formed. What is the empirical formula of this
compound?
![]()
A) CuCl
B) CuCl2
C) Cu2Cl
D) Cu2Cl3
E) Cu3Cl4
![]()
28. The compound shown below contains,

A) 8 sigma bonds and 2 pi bonds
B) 10 sigma bonds and 3 pi bonds
C) 8 sigma bonds and 3 pi bonds
D) 10 sigma bonds and 2 pi bonds
E) 6 sigma bonds and 5 pi bonds
29. Oxygen is produced by the reaction:
![]()
and collected over water. A sample of KClO3 produced 507 mL of O2(g) saturated with water vapor at 20ºC, where the vapor pressure of water is 17.54 mm Hg. The total gas pressure was 786 mm Hg. What mass of KClO3 decomposed? (Molar mass of KClO3 is 122 g/mol .)
A) 1.74 g
B) 1.78 g
C) 2.61 g
D) 2.73 g
E) 3.05 g
30. Which of the following statements about the vapor pressure of a liquid is false?
A) Vapor pressure is independent of the volume of liquid present.
B) Vapor pressure depends on the surface area of the liquid present.
C) Vapor pressure decreases as the intermolecular attractive forces in the liquid decrease.
D) Vapor pressure is independent of the standard heat of vaporization of the liquid.
E) Vapor pressure is the pressure exerted by the vapor, above a liquid, at a particular temperature.
APCBS Exam VI PAGE 8
The following two questions are short answer questions. Complete your answer in the space provided and turn in this page along with page 8 for grading.
(6) 31. Write the electron configuration for each of the following.
a) Mn 1s22s22p63s23p64s23d5
b) S2- 1s22s22p63s23p6
(4) 32. In the figure below Barometer #1 measures atmospheric pressure at 25 ºC. A sample of H2O(l) at the same temperature has been injected into Barometer #2. Use Barometer #3 to sketch what a barometer would look like which accurately depicts a liquid with a vapor pressure of 200 mm Hg at 25 ºC.

APCBS Exam VI PAGE 9
![]()
Name ________________________
AP Chemistry By Satellite
Exam Final
John I. Gelder
December 19, 1990
Exam #6 Multiple Choice Answer Sheet
![]()
Circle the most correct answer for each of the problems from the examination booklet. There is only one
correct answer for each question. Any question with more than one answer marked will be counted wrong. Your
score will be the total number of correct answers. So it is to your advantage to answer every question.
![]()
|
1. 2. 3. 4. 5. 6. 7. 8. 9. 10. 11. 12. 13. 14. 15. |
A A A A A A A A A A A A A A A |
B B B B B B B B B B B B B B B |
C C C C C C C C C C C C C C C |
D D D D D D D D D D D D D D D |
E E E E E E E E E E E E E E E |
16. 17. 18. 19. 20. 21. 22. 23. 24. 25. 26. 27. 28. 29. 30. |
A A A A A A A A A A A A A A A |
B B B B B B B B B B B B B B B |
C C C C C C C C C C C C C C C |
D D D D D D D D D D D D D D D |
E E E E E E E E E E E E E E E |
|||