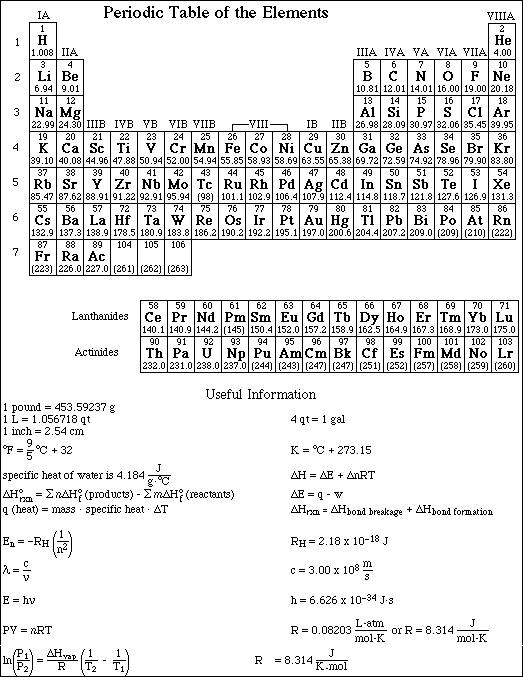Go to Main Index
Go to Main Index Go to Main Index
Go to Main Index
FINAL EXAM PART I
FALL 1991AP Chemistry by Satellite
The method used and steps involved in arriving at your answers must be shown clearly. This is important since you may obtain partial credit for doing so, while you will receive little or no credit if you do not. The last page contains a periodic table and important mathematical equations and constants.
PART A: Solve the following problem. (25 percent)
1a. Isopropyl alcohol, C3H8O, has a vapor pressure of 219.0 mmHg at 24.0 ºC. If the DHºvaporization is 19.3 kJ/mol , calculate the vapor pressure of isopropyl alcohol at 39.5 ºC.

b. What is the normal boiling point for isopropyl alcohol?
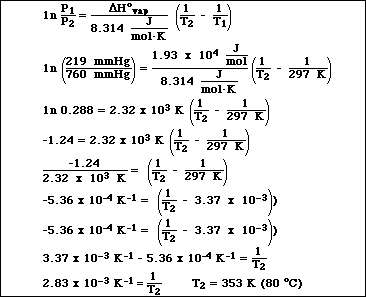
APCBS FINAL EXAM PART I PAGE 2
PART B: Choose ONE of the two problems in this part. (A second problem will not be scored.) (25 percent)
![]()
2a) A solution of potassium hydroxide was standardized by titrating with a sample of
potassium hydrogen phthalate, KHC8H4O4. (Note:
KHC8H4O4 is abbreviated as
KHP.) The reaction which occurs is;
![]()
A sample of KHP weighing 1.518 g was dissolved in water and titrated with the KOH solution. 26.90 mLs of the KOH solution were required to completely react with the sample of KHP. Calculate the molarity of the KOH solution.

(b) A 25.00 mL aqueous sample of H2CO3 was then titrated with the KOH solution. If 18.50 mLs of the KOH solution were required to completely neutralize the H2CO3, calculate the concentration of the H2CO3 problem your work must include the chemical equation which describes the solution. (Note: When solving this neutralization reaction.)

(c) How many grams of water are produced in the reaction in part b?
![]()
APCBS FINAL EXAM PART I PAGE 3
3a) A sample of a compound containing only carbon and hydrogen was analyzed and found to contain 85.7% carbon and 14.3% hydrogen. Calculate the empirical formula for the compound.


(c) Using the information in parts (a) and (b) of this problem, determine the molecular formula of the hydrocarbon.

![]()
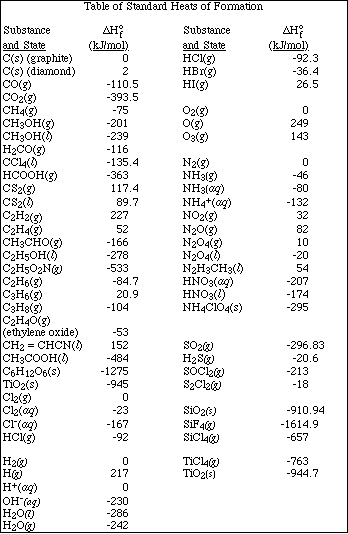
(d) If 7.00 g of the hydrocarbon are combusted in excess oxygen, calculate the moles
of CO2(g) and H2O(l)
![]()

APCBS FINAL EXAM PART I PAGE 4
PART C: Answer FIVE of the eight options in this part. (Answers to more than five options will not be scored.) (20 percent)
4. Write the appropriate formulas to show the reactants and products for the following. All solution are aqueous. For each reactant and product indicate the phase as (s)olid, (l)iquid, (g)as or (aq)eous. Balance the equation. In all cases a reaction occurs.
(a) Solutions of zinc sulfate and sodium phosphate are mixed.
![]()
(b) Excess hydrochloric acid is added to solid sodium carbonate.
![]()
(c) Hydrogen bromide gas is bubbled into a solution of sodium hydroxide.
![]()
(d) Magnesium metal is dropped into a solution of lead nitrate.
![]()
(e) A solution of hydrogen peroxide is heated.
![]()
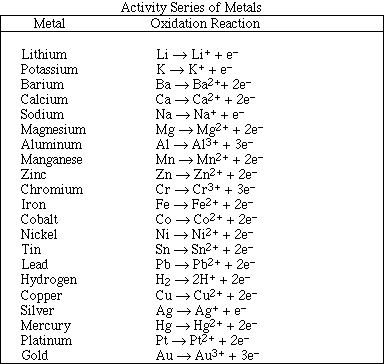
(f) A piece of potassium metal is dropped into a beaker of water.
![]()
(g) Pentane is burned completely in air.
![]()
(h) Solid zinc metal is added to dilute hydrochloric acid.
![]()
APCBS FINAL EXAM PART I PAGE 5
PART D: Select THREE of the following five topics. (Additional topics will not be scored.) (30 percent)
5a) Use a kinetic-molecular model to briefly describe the process of evaporation and how it occurs.
Evaporation is the process by which particles in the liquid phase escape and enter the vapor phase. Molecules can escape the attractions experienced by surrounding molecules when in the liquid phase; a molecule is on the surface of the liquid, has sufficient kinetic energy, and its escape direction is out of the liquid. Under these circumstances the particle is capable of overcoming the intermolecular attractive forces trying to keep it in the liquid phase and enter the vapor phase.
(b) Define the terms equilibrium vapor pressure for a liquid and normal boiling point for a liquid.
Equilibrium vapor pressure is the pressure exerted by the molecules
in the vapor phase, above a liquid in a closed container, at a particular
temperature.
Normal boiling point is the temperature at which the vapor pressure
of the liquid equals 1 atmosphere.
6a) Below are the Lewis structure and a ball-and-stick model of methyl alcohol. Indicate the geometry about both the carbon and the oxygen atom.

The geometry around the carbon atom is tetrahedral and bent around the oxygen atom.
(b) What are the important intermolecular attractive forces which occur in liquid methyl alcohol?
Hydrogen-bonding and London dispersion forces.
APCBS FINAL EXAM PART I PAGE 6
(c) Draw several methyl alcohol molecules and clearly indicate how adjacent molecules interact. In your sketch indicate the most important intermolecular attractive force between adjacent methyl alcohol molecules.
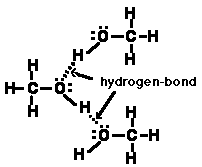
7a) Define ionization energy, electron affinity and electronegativity.
Ionization energy (first) is the energy required to remove an electron from a neutral atom in the gas phase.
![]()
Electron affinity is the energy change that occurs when an electron is added to a neutral atom in the gas phase.
![]()
Electronegativity is a measure of the attraction an atom has for the electron pair in a covalent bond.
(b) Indicate the direction of increasing ionization energy and electronegativity, going across a period and down a group.
The ionization energy decreases moving down a group and increases moving across (left-to-right) a period.
The electronegativity decreases moving down a group and increases moving across (left-to-right) a period.
8. List and describe two techniques that could be used to separate the components of a heterogeneous mixture.
Assume the heterogeneous mixture contains a liquid and a solid. Filtering: The heterogeneous mixture is poured onto a paper filter which has been folded and placed into a funnel. The solid component of the mixture does not pass through the filter paper, while the liquid component does. Filtering is generally used when the solid is off small particle size and dispersed throughout the liquid. The mixture has a cloudy appearance.
Decanting: When the solid particles are large they settle to the bottom of the container and the liquid component of the mixture can be separated by pouring it carefully off the solid.
APCBS FINAL EXAM PART I PAGE 7
9a) Draw the Lewis electron-dot structures for CH4, NH3 and H2O.
![]()

(b) Predict the molecular geometry and H-X-H bond angle (where X is C, N or O) for
each of the molecules. Explain how you arrived at your predictions.
In methane, CH4, the bond angles are all 109.5 º. The carbon atom is sp3 hybridized and the molecular geometry is tetrahedral.
![]()
In ammonia, NH3, the bond angles are 107º. The bond angle
is depressed due to the repulsion of the bonding electrons and the
nonbonding lone pair. The lone pair of electrons occupies a large
volume of space so the bonding pairs of electrons feel some
repulsion and they are forced closer together.
![]()
In water, H2O, the bond angles are about 105º. The angle is
even smaller due to the presence of two lone pairs of electrons on the
oxygen atom. The repulsion experienced by the bonding pairs is
even greater.