

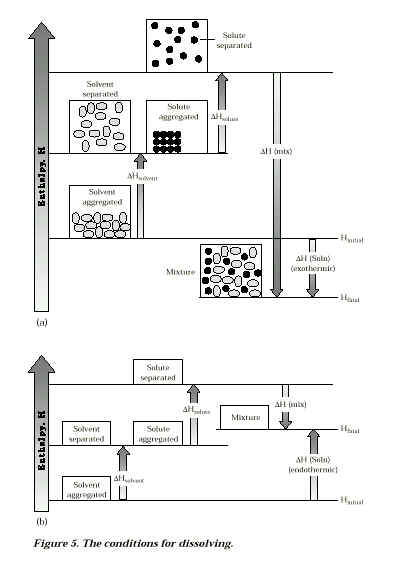
DHsolute + DHsolvent + DHmixing = DHsolution
If DH in "A" is GREATER than DH in "B," then
- DHsolution is Endothermic
- Solution gets colder as it forms
- Heating will cause more solid to dissolve
- Energy needed to break old attractive forces > Energy released by formation of new forces
If H in "A is LESS than DH in "B," then
- DHsolution is Exothermic
- Solution gets warmer as it forms
- Heating will decrease the amount of solid dissolved
- Energy needed to break old attractive forces < Energy released by formation of new forces
There is a tendency for entropy to increase when solutions form.
IF DS is positive, solution is favored.
IF DS is large enough, it can overcome DH effects and substance will go into solution.These conditions for dissolving can also be represented by the following charts.
(page 11,12,13)