Laboratory Activity: Teacher Notes
Continued
Anticipated Student Results
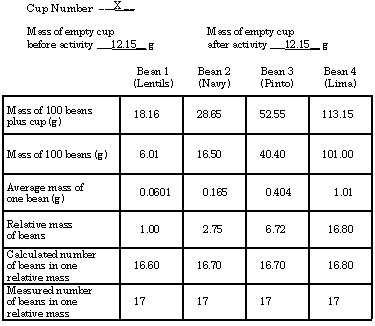
These values are typical student values. Lima beans vary greatly in size, thus having the largest uncertainty.
Answers to Implications and Applications
- The calculated number of beans in one relative mass stayed the same at 16.7 ± 0.1 bean. The measured number stayed constant at 17 ± 1 bean.
- The lima bean relative mass is about 17 times larger than the lentil bean relative mass. There are 17 beans in a relative mass. These values are the same.
- The relative mass is the ratio of the mass of one type of bean to the mass of another type of bean. This mass ratio insures that when we weigh beans in this mass ratio, we must obtain the same number of beans. (Since a lentil bean is only 1/17 as massive as the most massive bean measured-the lima bean-there must be 17 beans in a relative mass.)
- The volumes of the relative mass piles are not the same. Even though each pile has the same number of beans, they have different sizes.
- The average mass of the least massive bean is 0.0601 g. The relative mass of the least-massive bean is 1.00.
- If it is the least massive element, its relative mass should be 1.00.
Part II
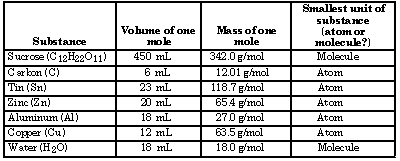
Note: These are not molar volumes, but the approximate volumes occupied by 1 mole of each substance, eg. crystalline sucrose.
- Sucrose occupied the largest volume. Carbon occupied the smallest volume.
- Sucrose had the largest molar mass and the most massive individual particles. These answers must be the same because all beakers contained the same number of individual units.
- One mole of various entities have different masses because their individual particles have different masses, just as different kinds of beans have different masses.
- One mole of various entities occupies different volumes because their individual particles have different volumes, just as piles of relative masses of beans have different volumes. (The volumes of solids that are not a single crystal, however, will not be directly related to the volume of the atoms or particles.)
- The fastest way to obtain a relative mass of beans would be to count the beans. The fastest way to obtain a mole of beans would be to weigh them. (At least in principle. The mass of a mole of beans would be incredibly large- on the order of 10 22 g.)
Part III
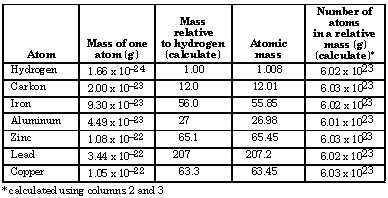
- All atomic masses agree with the relative masses to three significant figures. (Some variation in the last significant digit is always expected.
- Atomic masses are relative masses. They are calculated relative to some reference atom. NOTE: Molar masses of elements are accurately known. The number of carbon atoms in a unit cell is determined precisely by X-ray crystallography, and the density of carbon is determined. Using these precise measurements, all other atomic masses are taken relative to the carbon-12 isotope as exactly 12.
- The number of atoms in a relative mass is constant at 6.02 x 10 23 .
- This is Avogadro's number.
- There are 6.02 x 1023atomic mass units in one gram.
- A mole of these atoms would have a mass of 197 g. (TEACHER NOTE: This is a gold atom.)
Continue