

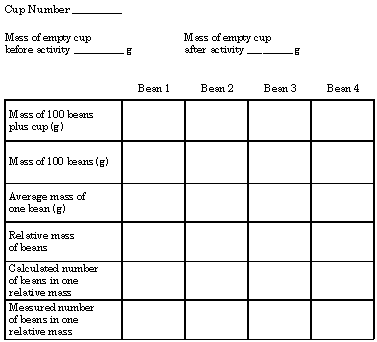
Implications and Applications
Part II
Examine the beakers of elements and compounds on display. Each beaker contains one mole of a particular substance. Record information on the chart below.
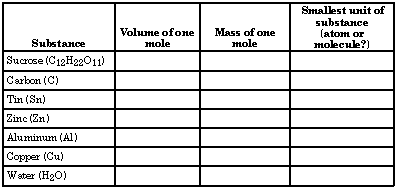
Part III
Below is a chart reporting the average masses of individual atoms. Calculate the relative mass of each element and record it in the chart. Then look up the molar mass (atomic mass) of each element on a Periodic Table and record it in the table.
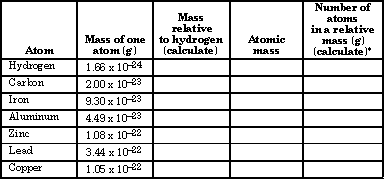
* Although this calculation works with a relative mass of hydrogen of 1.00 as its basis, atomic masses are actually calculated as relative to a particular type of carbon atom viewed as exactly 12.00.
 |
TABLE OF CONTENTS | TOPIC OVERVIEW | CONCEPT/SKILLS DEVELOPMENT | LINKS/CONNECTIONS | EXTENSIONS |  |
|---|