Inorganic Qualitative Analysis (QUAL)
27
Appendix
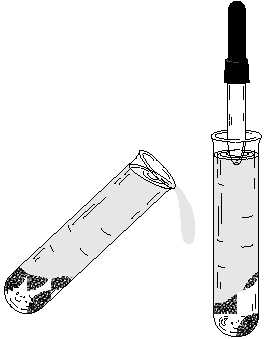
Decanting
This is the term used to denote removing a supernatant liquid from a precipitate. There are two
decanting methods. The first is to simply pour the spn out of the test-tube leaving the ppt behind.
The second method is to draw the spn out of the test-tube using a dropper. This dropper method
is used when the ppt is not a firm pack and might pour out with the spn. It is also the preferred
decantation method where the spn is to be saved for later use. It is often difficult to pour directly
from one small test-tube to another.
When using the dropper method be
sure to compress the bulb before
insertingthedropper.Arushofbubbles
in the spn will cause the ppt and spn to
remix.
Figure B. (a) Effect of
centrifuging and (b)
decantingbypouringor
drawing.
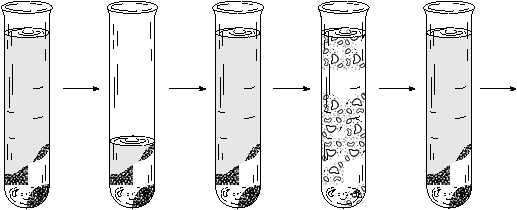
Washing a Precipitate
Not all of the supernatant is removed from the test-tube when one decants. A few drops remain on top of the
precipitate, some clings to the test-tube walls and some is trapped within the packed precipitate. If a later test
on the ppt requires that the chemicals in the spn be absent, these traces of spn are removed by a procedure called
washing.
Washing a ppt involves the following sequence of steps. (1) Decant the original spn. (2) Add to the ppt about 1 mL
of distilled water (or other prescribed liquid). (3) Completely mix the H2O and ppt. This allows the H2O to leach
out any spn trapped in the packed ppt. (4) Centrifuge the mixture. (5) Decant the liquid, discarding it. (6) Repeat the
process. Usually two such water washes are sufficient as they will leach out about 97% of the spn originally left
in the test-tube.
Figure C. Washing a precipitate
(a)
Decant
+H2O
Mix
Centrifuge
Repeat