Major Chemical Concept
The products of a double replacement reaction can be determined experimentally. The amounts of reactants are equimolar.
Level
General or basic student.
Expected Student Background
Students should be familiar with filtration and be able to use a burner safely.
Time
45 min
Safety
Read the Safety Considerations in the Student Version.
Materials (For 24 students working in pairs)
Nonconsumables
Consumables
20 min to assemble the materials.
Pre-Laboratory Discussion
Review the evidence for chemical change and double replacement reactions. Emphasize that the only way we can be sure about the identity of products is to test them. Discuss flame tests if students are unfamiliar with them. The only use of the flame test in this activity is to identify potassium and calcium ions.
Teacher-Student Interaction
During the activity focus students' attention on the difference in properties of the reactants and products. Caution them to observe the flame test before the splint catches on fire. They should compare the colors of the flame tests on the known starting materials and the products.
Anticipated Student Results
| Test | 1 | 2 | 3 | 4 |
| Flame | Violet | Red | Violet | Orange |
| Acid | Bubbles | No Rxn | No Rxn | Bubbles |
Answers to Implications and Applications
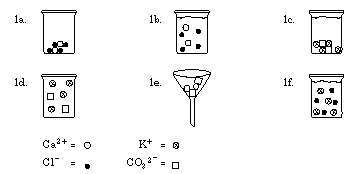
2. K 2 CrO 4 (aq) or Na 2 CrO 4 (aq)
3. Make a solution of a small portion of each solid. Test each solution with a solution of calcium chloride. The one that precipitates is K 2 CO 3 . Alternatively, add dilute hydrochloric acid to both. The one that forms bubbles is K 2 CO 3 .
The discussion should focus on student answers to Data Analysis and Concept Development questions. The Pictures in the Mind section is particularly important in helping students develop the concept of the particulate nature of matter. These pictures can also be used to explain anomalous results arising from incomplete separations.
Extensions
Assessing Laboratory Learning
 |
TABLE OF CONTENTS | TOPIC OVERVIEW | CONCEPT/SKILLS DEVELOPMENT | LINKS/CONNECTIONS | APPENDEX |  |
|---|