Acids and bases are chemical species that exhibit distinctive sets of observable properties. Acids taste sour (like vinegar and lemon), cause blue litmus to turn red, liberate hydrogen gas when they react with certain metals (like iron, zinc, and aluminum), and neutralize bases. Bases taste bitter (like soap), feel slippery to the touch, cause red litmus to turn blue, and neutralize acids.
2.Conceptual Definitions
Acids and bases can be defined conceptually to help account for what is
happening on a microscopic level.
Arrhenius concept. An acid is a substance that, when dissolved in
water, forms hydrogen ions (or protons, H+). A base is a substance
that, when dissolved in water, forms hydroxide ions (OH-). The
Arrhenius concept is limited in several ways. Hydroxide ion is singled out as
the only source of base character. However, other species can display basic
properties (for example, ammonia). The hydrogen ion cannot exist alone in water solution. It is chemically bonded to water to form what is conventionally written as the hydronium ion, H3O+. In fact, the hydronium ion is associated through hydrogen bonding with a variable number of water molecules (for example, [H9O4]+, in which H3O+ is associated with three water molecules). Moreover, the Arrhenius concept does not cover acid-base reactions in non aqueous solvents.
Brønsted-Lowry concept. An acid is a proton donor; a base is a proton acceptor. Any Arrhenius acid is also a Brønsted-Lowry acid and any Arrhenius base is also a Brønsted-Lowry base. Hydrochloric acid (HCl) is an Arrhenius acid and is therefore, a Brønsted-Lowry acid. As a Brønsted-Lowry acid, HCl can donate a proton to water.

Magnesium hydroxide, Mg(OH)2, is an Arrhenius base and therefore also a Brønsted-Lowry base. As a Brønsted-Lowry base, OH- can accept a proton:

Ammonia, , can also serve as a Brønsted-Lowry base, because it can accept a proton from water:
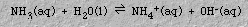
Water can function both as a Brønsted-Lowry acid (Equation 2) and a Brønsted-Lowry base (Equation 1). The Brønsted-Lowry concept of acids and bases is more general than is the Arrhenius concept. In the Brønsted-Lowry concept a base accepts protons (OH- is only one example). Brønsted-Lowry acids and bases can be ions (for example, OH-) or molecules (for example, NH3 and H2O). Some species can act as either acids or bases, depending on the nature of the other reactant (for example, H2O). Furthermore, Brønsted-Lowry acid-base reactions are not restricted to aqueous solution.
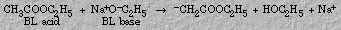
3. When a suitable amount of base is added to an acid solution, the base and acid properties disappear and the acid is said to be neutralized. A neutralization reaction is the reaction of an acid and a base that results in an ionic compound and possibly water. The ionic compound is called a salt.

