-
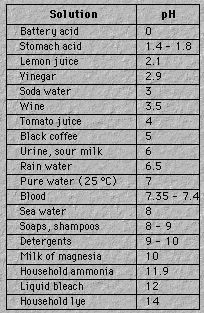
For coffee it's 5, for tomatoes it's 4;
While household ammonia is 11 or more.
It's 7 for water, if in a pure state,
But rain water is 6, and sea water is 8.
It's basic at 10, quite acidic at 2,
And well above 7 when litmus turns blue.
Some find it a puzzlement. Doubtless their fog
Has something to do with that negative log.
.
.
.
.
.
.
.
.
.
.
.
.
Figure 19. Approximate pH
of some common solutions.
.
8. Remember the color litmus becomes in acidic and basic solutions:
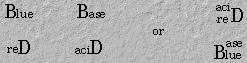
9. Generally, concentrations of acidic and basic solutions are expressed as moles per liter (M). Commercial acids and bases are sold as percent by mass. For example, concentrated hydrochloric acid is 37% by mass. The concentration of sulfuric acid in rain water is measured in parts per million (ppm).
10. Practice writing balanced equations for neutralization reactions.
11. Review safety precautions when handling acids and bases.


