Concept/Skills Development
Counterintuitive Examples and Discrepant Events
- Diluting a weak electrolyte (HC2H3O2) with water increases the
electrical conductivity. (See Suggested Laboratory Activity, Strong vs.
Weak Acids)
- Carbon dioxide bubbled through limewater causes a precipitate to form.
Continued bubbling causes the precipitate to disappear. (See Underground
sculpture, ChemMatters (1984),1(2), pp. 10-11.
- Some active metals react with either acids or bases to produce hydrogen
gas. (For example, aluminum will react with either hydrochloric acid or sodium
hydroxide, releasing hydrogen gas. See Suggestions for Other
Demonstrations, Making hydrogen gas from an acid and a base.)
- The same amount of hydrogen gas will be produced when a sample of an active
metal is added to equal volumes of concentrated acid solution and dilute acid
solution (if the metal is the limiting reactant).
- Bicarbonate salts can be used to neutralize either an acid or a base. This
can be illustrated by the following equations:
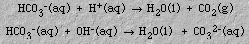
Metaphors and Analogies
- [H+] vs. pH: a see-saw relationship (see
Pictures in the Mind below); one goes up (increases) while the other
goes down (decreases).
- A proton shifting from an acid to a base can be likened to a baseball
being thrown from a pitcher (the acid) to a catcher (the base).
- Universal indicator color changes follow the colors in the rainbow as the
pH moves from 2 to 10. The name ROY G BIV helps keep the colors straight: Red, Orange, Yellow, Green, Blue, Indigo, Violet. A pH of 7 produces a yellow-green hue.
Pictures in the Mind
 1. Ionization. Graphical pictorial representation of the behavior of
acids of different strengths in aqueous solution.
1. Ionization. Graphical pictorial representation of the behavior of
acids of different strengths in aqueous solution.
Acids and Bases
(Page 21)
 1. Ionization. Graphical pictorial representation of the behavior of
acids of different strengths in aqueous solution.
1. Ionization. Graphical pictorial representation of the behavior of
acids of different strengths in aqueous solution.
