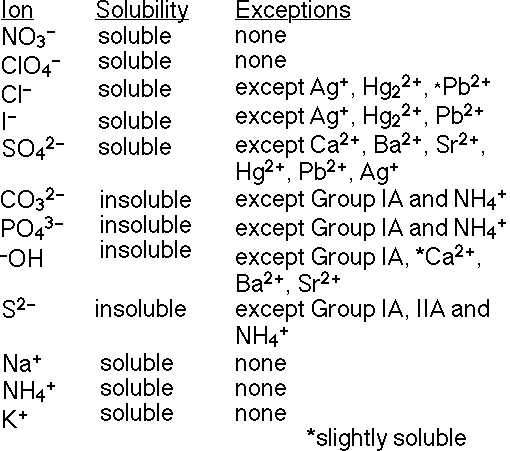Double replacement reactions
A reaction between two soluble ionic compounds producing two
new ionic compounds where the cations and anions have exchanged 'partners'.
You must use the Solubility Table to predict solubility. (Remember you will
need to be able to write the ionic and net ionic equations.)
Na2SO4(aq) + CaCl2(aq) --->
CaSO4(s) + 2NaCl(aq)
2AgNO3(aq) + Na2CO3(aq) --->
Ag2CO3(s) + 2NaNO3(aq)
2HCl(aq) + Na2CO3(aq) --->
CO2(g) + 2NaCl(aq) + H2O(l)
Here are those same reactions as they should be written by an
AP student...(NOTE: phases are not required)
SO42-(aq) + Ca2+(aq) --->
CaSO4(s)
2Ag+(aq) + CO32-(aq) --->
Ag2CO3(s)
2H+(aq) + CO32-(aq)
---> CO2(g) + H2O(l)
Solubility Table