![]() Pre-lab Questions
Pre-lab Questions
![]() Experiment
Experiment
![]() Post-lab Questions
Post-lab Questions
![]() Top
Top
EXPERIMENT 3: STOICHIOMETRY Procedure A: Gravimetric and Quantitative Measurements using Barium Sulfate
![]()
(a) precipitate
![]()
(b) digestion
![]()
![]()
![]()
(a) How many moles of magnesium were placed in the crucible?
![]()
(b) What element did the magnesium react with during heating?
![]()
(c) How many moles of the element combined with the magnesium?
![]()
(d) What is the empirical formula of the compound?
![]()
Procedure A: Gravimetric and Quantitative Measurements using
Barium Sulfate
![]() Top
Top
| EQUIPMENT: | ||
| laboratory balance ....................1 | drying oven ................optional | rubber policeman ......... 1 |
| beaker, 50 mL ........................3 | filter paper..................5 sheets | spatula ..................... 2 |
| beaker, 100 mL.......................2 | flask, Erlenmeyer, 250 mL...1 | stirring rod ................ 1 |
| beaker, 250 mL.......................2 | forceps..........................1 | test tube, 13 x 100 mm .. 2 |
| Bunsen burner ........................1 | funnel, polyethylene ..........1 | thermometer, ºC .......... 1 |
| rubber tubing ..................... | weighing paper ............8 sheets | wash bottle ................ 1 |
| matches............................ | pan (metal or plastic)..........1 | watch glass, 3 in.......... 1 |
| cylinder, graduated, 10 mL .........1 | paper towels....................3 | watch or clock ............ 1 |
| cylinder, graduated, 50 or 100 mL.1 | ring..............................1 | wire gauze................. 1 |
| dropper, medicine ....................1 | ring stand.......................1 |
![]()
Before you begin the experiment you will watch a videotape demonstrating several
techniques which you will find important. Use the space below for your notes covering these
procedures. Your instructor will read your notes, so be complete.
![]()
![]()
The goal of this experiment is to determine the formula of a compound, barium sulfate. The compound will be formed as a precipitate in a chemical reaction. The amounts of reactants will be varied in order to see if this changes the formula of the product. You will determine how many compounds there are that contain only barium ions and sulfate ions. In the process, you will practice several important quantitative laboratory techniques. You will also do practical calculations involving limiting reagents. This experiment is divided into two parts. In Part I you will practice the techniques necessary to obtain quantitative yields of a precipitate. In Part II you will apply the same procedure to a series of experiments in order to determine the chemical formula(s) of barium sulfate. Each student or group of students will preform slightly different experiments and share the results with the rest of the class so conclusions can be based on the largest possible amount of data. You will asked to perform four separate experiments. During the procedure you must often wait 10-20 minutes for filtering, cooling or drying of the product to be completed. It is highly advisable that you begin the next experiment during this time.
When you have completed Part I consult with your instructor about the amounts of barium nitrate and sodium sulfate you are to use in the additional experiments. Record the names of the members of the group performing each experiment in the space provided in Part II. Be sure to share your data with each of your classmates and to obtain from group members the data for which they are responsible. To assist in this sharing of data, an overall data table has been included at the end of the experiment.
Fold a sheet of weighing paper in half and then in fourths. Unfold and carefully weigh the paper on an analytical balance. Record the mass in the table (Obs. #1) below. Add approximately 0.4 g of barium nitrate with a clean dry spatula and reweigh. (Do not use less than 0.390 g or more than 0.410 g of barium nitrate.) Be careful not to spill any chemicals. If you do, clean it up at once! Record the mass in the table.
(HAZARDS: Barium nitrate is poisonous. Because of its solubility in water, it can be absorbed readily. Use caution when preparing and handling aqueous solutions of this substance. In the event of contact, rinse liberally with deionized water. Swallowing this compound may be fatal.)

![]()
![]()
Carefully transfer the barium nitrate to a clean 250 mL beaker and add 50 mL of deionized water. Stir with a clean glass rod until all of the solid dissolves. Do not remove the rod from the beaker! Describe the appearance of the barium nitrate before and after adding it to the water.
![]()
Measure 10 mL of concentrated (12 M) hydrochloric acid in a small graduated cylinder.
The acid should be located in the fume hood because of its strong vapors. Add 1 or 2 mL of acid
to the beaker in the hood. Stir the solution with a clean glass rod. Continue to add the acid to the
solution of barium nitrate in the beaker a few milliliters at a time. Stir between additions. Do not
remove the beaker from the hood until all of the acid has been added. Use deionized water from a
wash bottle to rinse off the glass rod so that all of the dissolved ions fall back into the beaker
before you remove the rod from the beaker. (The hydrochloric acid is needed to prevent the
formation of barium hydroxide.)
(HAZARDS: Concentrated solutions (6-12 M) of HCl can cause severe burns and permanent damage to the eyes. Inhalation can cause coughing and choking. If you should come in contact with either solution, rinse the area liberally with water and notify your instructor.)
Using a new sheet of weighing paper accurately weigh out approximately 0.8 g of sodium sulfate and record the results in Obs. #3. (Do not use less than 0.790 g or more than 0.810 g of sodium sulfate.)

![]()
Transfer the sodium sulfate to a 100 mL beaker. Add 50 mL of deionized water and stir
with a clean glass rod until all of the solid dissolves. Again, be sure to wash all of the dissolved
ions off the stirring rod before you remove it from the solution. Describe the appearance of the
sodium sulfate before and after adding it to the water.
![]()
![]()

![]()
(HAZARDS: Barium sulfate is very poisonous if swallowed, inhaled
or absorbed through the skin. Because of insolubility in water,
absorption through the skin is unlikely. Use caution when
handling.)
![]()
Describe your observations upon addition of the sodium sulfate to the warm barium nitrate
solution.
![]()
During the heating, a process called digestion occurs. The tiny crystals of precipitate that
were initially formed when the solutions were mixed re-dissolve. These crystals were formed very
rapidly and might contain impurities. Crystals re-form much more slowly in the hot solution.
They tend to be larger. The larger size makes them easier to filter. Because impurities are most
often found on the surface of crystals, and fewer large crystals have less surface area than many
tiny crystals, digestion increases the purity of the precipitate.
After heating, carefully move the beaker to the lab bench. Do not agitate the solution, but let the precipitate settle to the bottom of the beaker. Prepare an ice bath by filling a plastic or metal pan half full of ice and adding a little water. After the beaker has cooled for 15 to 20 minutes, carefully set the beaker in the ice bath and allow it to cool for about 10 minutes or until the solution
![]()
![]()
inside is at or below room temperature. Do not move the hot beaker directly from the flame to the ice bath. The beakers will break without the initial cooling period! While the beaker is cooling, wash and dry your hands thoroughly. Obtain 2 pieces of filter paper. Label one C for control and the other with the experiment number (assigned by the instructor) very near the edge of the paper using a pencil. Fold the filter papers into a cone shape as shown in the prelab videotape and weigh on the labortory balance. Record the mass of the control filter paper in Obs. #6 and the mass of the second piece of filter paper in Obs. #8.

Set the control paper in a funnel and the funnel in a 250 mL Erlenmeyer flask. Slowly pour about 100 mL of deionized water through the filter paper. When all the water has passed through the funnel, gently remove the paper with forceps and place it in a clean 50 or 100 mL beaker. Pour the water out of the flask and replace the funnel in the 250 mL Erlenmeyer flask. Do not repeat this part of the procedure for each experiment. You will need only one control. The control filter paper must be dried in a manner identical to the other filter papers in order to assure consistent and meaningful results, so set the control aside until the experiment is complete.
(Note: While you are waiting for the solution to cool, which will take a minimum of 35 minutes, you may begin the the next experiment you are to run. Ask your instructor which experiment(s) you are to do.)
Place the second weighed, folded filter paper in the funnel. When the 250 mL beaker and contents have cooled, wet the filter paper with a little deionized water. Rinse the bottom of the watch glass and walls of the beaker with deionized water and drain washing into the beaker. Carefully pour most of the water through the filter. Use a rubber policeman to transfer the precipitate to the filter paper. Being careful not to use too much water (some of the precipitate will dissolve), use a jet of deionized water from a wash bottle to rinse any remaining precipitate into the filter funnel. Rinse the rubber policeman with deionized water. It is extremely important that all of the precipitate is transferred into the filter.
Once the water has drained, carefully remove the filter paper from the funnel with your forceps and place it in a clean 50 or 100 mL beaker. Forceps are necessary because of the hydrochloric acid which has soaked into the filter paper. If any solid is visible in the flask, this must be collected on another piece of filter paper - consult your instructor. Carefully decant a few milliliters of the filtrate into each of two clean test tubes. To one of the test tubes add a few drops of barium nitrate solution. To the other test tube add a few drops of sodium sulfate. Describe your observations.
![]()
![]()
+ If control has gained weight subtract this amount from each observed mass of barium sulfate. If control has lost weight, add this amount to each observed mass of barium sulfate.
![]()
From your description in Obs. #7, determine which chemical was present in excess and explain how you arrived at your conclusion. Record the name of the excess ion in Table I (on the calculation page) for each experimental run.
![]()
Dispose of the filtrate and test solutions. All solutions containing barium ions must be
disposed of in a properly labeled waste bottle.
After you have completed your filtration, the filter papers must be dried and reweighed in order to determine the mass of barium sulfate precipitated. There are two ways to do this. Ask your instructor which method you should use. The control filter paper must be dried in the same way as the experimental filter papers in order to insure consistent and meaningful results.
1) Carefully store the labeled filters until next laboratory period, when they should be dry and ready to weigh. (Up to one week may be required for drying.) 2) Write your name on the white dot on the outside of the beakers containing the filter papers. Place the beakers in an oven at about 110ºC for approximately 20 minutes. Carefully remove the beakers (they will be very hot) and allow them to return to room temperature. This will take 10 to 20 minutes. When cool, the filter papers will be ready to weigh.
The final mass of the control filter paper should be recorded in Obs. #6 (on the previous page), and the others in Obs. #8 (below).
If time permits, repeat the procedure with a second sample. (It is not necessary to prepare a second control filter). Record the data in the blanks for Exp. #2.
Note: Each time you begin the procedure, it is important that your glassware be clean. It may be wet with deionized water, but it should not contain any other material.

![]()
![]()
Why is it necessary to have a control piece of filter paper?
After completing the procedure for the first two experiments your instructor will assign one or two additional experiments using the masses of barium nitrate and sodium sulfate listed in the table below.

Record the assigned experiment number(s) in the appropriate column in Obs. #1, #3 and #8. Obtain the names of the other members of your class and which experiment(s) they are responsible for and record them below. You will share data with these students to complete Tables I, II and III on following pages.

Repeat the experimental procedure for the two additional experiments and determine the mass of barium sulfate produced. Report the data to the other members of your class.
![]()
![]()
CALCULATIONS
Copy the data you have recorded in Observations #1, #3, #7, and #8 into Table I.
Complete the italicized columns for each experiment you performed. Share this data with your
classmates. Obtain from them the information to complete the italicized columns for the
experiments for which they were responsible. (Data in the italicized columns are measured directly
in the experiment. Only this information needs to be shared in the groups. All other columns can
be calculated from the information in the italicized columns.)
Given the following information, complete Tables I, II and III using your data and that
collected by your classmates. Instructions for the necessary calculations and space for sample
calculations is included following Table III.

![]()
![]()
Table III

Sample Calculations: (Show a sample of each calculation used to complete the tables above.)
Moles of Sulfate Ion: Calculate the moles of sulfate ion present in the sodium sulfate, given that each sodium sulfate unit contains one sulfate ion.
![]()
Moles of Barium Ion: Calculate the moles of barium ion present in the barium nitrate, given that
each barium nitrate unit contains one barium ion.
![]()
Limiting Ion: Determine which ion is the limiting reagent, based upon the observed excess ion.
![]()
Moles of limiting Ion: Fll in the number of moles previously calculated for the limiting ion.
![]()
![]()
Mass of Limiting Ion: Calculate the mass in grams of the limiting ion present in the initial sample using the moles of limiting ion and the formula mass of the ion.
![]()
Mass of excess ion in product: Knowing the mass of the product and the mass of the limiting ion,
calculate the mass of the other ion present in the product.
![]()
Moles of excess ion in product: Convert the mass of excess ion present in the product into moles.
![]()
![]() available to react: Calculate the ratio of ions present in the initial sample.
available to react: Calculate the ratio of ions present in the initial sample.
![]()
![]() in product: Calculate the ratio of ions present in the product.
in product: Calculate the ratio of ions present in the product.
![]()
![]()
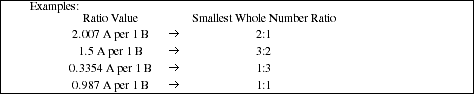
![]()
![]()
![]()
![]()
![]()
![]()
Contents
![]() Return to
Return to![]() Index of Experiments
Index of Experiments
![]()