Go to Main Index
Go to Main Index Go to Main Index
Go to Main Index
Name ________________________
AP Chemistry By Satellite
John I. Gelder
Exam II
March 7, 1991
![]()
INSTRUCTIONS:
1. This examination consists of a total of 10 different pages. The last two pages include important mathematical equations and constants, a periodic table and tables of acid and base dissociation constants. All work should be done in this booklet.
2. PRINT your name, high school, teaching partner's name and today's date now in the space at the top of this sheet. DO NOT SEPARATE THE PAGES.
3. Answer all questions that you can and whenever called for show your work clearly. Your method of solving problems should pattern the approach used in lecture. Clearly state any assumptions used when solving equilibrium problems. You do not have to show your work for the multiple choice or short answer questions.
4. No credit will be awarded if your work is not shown in problem 3 - 9. Please circle your final answer!
5. Point values are shown next to the problem number.
6. Budget your time for each of the questions. Some problems may have a low point value yet be very challenging. If you do not recognize the solution to a question quickly, skip it, and return to the question after completing the easier problems.
7. Look through the exam before beginning; plan your work; then begin.
![]()
8. Relax and do well.
![]()

APCBS Exam II 2
![]()
(4) 1. The reaction
![]()
![]()
has been carefully studied at 25 ºC and Kc for the reaction is 0.00851. Which direction will the reaction
proceed to establish equilibrium under each of the following initial conditions?
![]()
i) [NO2]o = 0.100 M : [NO]o = 0 : [O2]o = 0
![]()
The reaction will proceed from left to right.
![]()
ii) [NO2]o = 0.100 M : [NO]o = 0.0450 : [O2]o = 0.0450
![]()

![]()
(9) 2. Complete the following table

APCBS Exam II 3
![]()
(12) 3. Given the reaction
![]()
![]()
Initially (before any reaction occurs) a 1.00 liter reaction vessel at 25 ºC contains NOBr at a partial
pressure of 300 mm Hg and no nitrogen monoxide or bromine. After equilibrium is established the
partial pressure of Br2 is 45.0 mm Hg. Calculate Kp for the reaction.
![]()

APCBS Exam II 4
![]()
(12) 4. The equilibrium constant, Kc, for the reaction
![]()
![]()
is 7.31 at 300 K. If a 1.50 mol sample of H2O and a 1.50 mol sample of CO are introduced into a
10.0 liter vessel, calculate the equilibrium concentrations of all species.
![]()

![]()
After equilibrium is established, 0.0750 mol of H2 and 0.075 mol of CO2 are added to the reaction
vessel. Which direction (L ![]() R or L
R or L ![]() R) will the reaction proceed to re-establish equilibrium?
R) will the reaction proceed to re-establish equilibrium?
The reaction will proceed from right to left, L ![]() R.
R.
What will be the new equilibrium concentrations of all species at 300 K?

APCBS Exam II 5
![]()
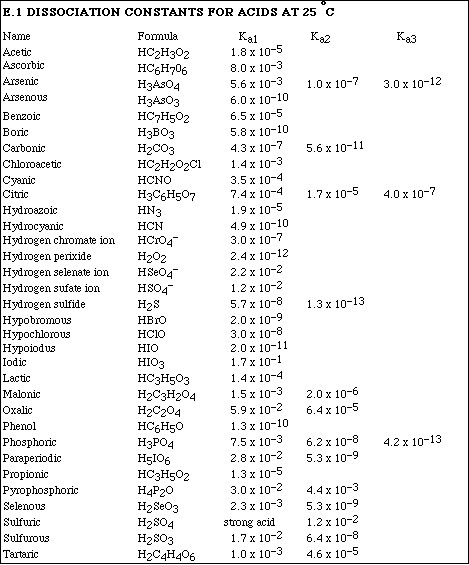
(12) 5. Calculate the pH of a 0.250 M solution of propionic acid, HC3H5O2.

![]()
(12) 6. Calculate the pH of a 0.700 M solution of C6H5NH2.
![]()

APCBS Exam II 6
![]()
(10) 7. Calculate the pH of a 0.430 M solution of NaC7H5O2.
![]()

![]()
(10) 8. Malonic acid, H2C3H2O4, is a diprotic acid. Calculate the pH of 0.500 M solution of malonic acid.
![]()

APCBS Exam II 7
![]()
(10) 9. The equation which describes the decomposition of dinitrogen monoxide is,
![]()
![]()
The reaction follows 2nd order kinetics at 900 K. In a particular experiment the concentration of N2O
falls from 0.250 M to 0.195 M in 100 s. Calculate the concentration of N2O after 200 s.
![]()

APCBS Exam II 8
![]()
Multiple Choice: (9 points)
Print the letter (A, B, C, D, E) which corresponds to the answer selected.
10. D ![]() 11. E
11. E ![]() 12. D
12. D
![]()
ONLY THE ANSWERS IN THE AREA ABOVE WILL BE GRADED. Select the most correct answer
for each question. Each question is worth 3 points.
10. Which of the following solutions has the lowest pH?
a) 0.500 M HC2H3O2
b) 0.500 M NaCN
c) 0.500 M NH3
d) 0.500 M HN3
e) 0.500 M H2S
11. Calculate the initial concentration of a sample of acetic acid if the pH of the solution is 4.85.
a) 4.85 M
b) 0.100 M
c) 1.1 x 10-5 M
d) 1.4 x 10-5 M
e) 2.5 x 10-5 M
12. When 1.00 gram of an unknown strong acid is dissolved in 100. mLs of water the pH of the solution is 0.908. Assuming no change in volume when the solution is formed calculate the molar mass of the unknown acid.
a) 20.0 g/mol
b) 36.5 g/mol
c) 63.0 g/mol
d) 80.9 g/mol
e) 100 g/mol