In Chapter 7 we established the theoretical organization to
the periodic table, based on our knowledge of the electronic
arrangement of multi–electron atoms. So what's next?
It turns out that as interesting as our quantum mechanical
model of the atom is; there is more. If all we studied were the
electronic arrangements of atoms, chemistry would not be as
interesting as it is. Because the core of chemistry is interested
in the thousands of combinations and permutations which can be
made from the hundred–odd elements in the periodic table. We
most commonly encounter the elements, not as pure substances, but
as constituent parts of compounds. They can be simple like water
(H2O), carbon dioxide (CO2) and sodium
chloride (NaCl). Or complex as in proteins, nuclei acids or fatty
acids. However, simple or complex all compounds are formed of
aggregates of atoms combined in some characteristic ratio.
When Dalton first formulated his basic ideas on bonding 150
years ago, he was uncertain of the nature of the attractive
forces which held atoms together. At first only naive pictures of
hooked atoms interconnected with each other were used. Our
present model really did not evolve until an understanding of the
illucidation of the atomic structure of the atom. It is from this
vantage point that we're able to gain the perceptions needed to
view the combination of chemical elements.
Our present model of bonding is very sophisticated, but has
its basis on the interaction of the valence electrons on atoms.
Inner-core electrons feel large attractions to the nucleus, so it
is only the valence electrons that become involved in bonding.
The number of valence electrons associated with the atom of a
representative element (any Group with an 'A' deignation) is
obtained from its Group number. For example, elements in Group IA
have one electron in their outer-most orbital, and have one
valence electron. Group VIIA elements, the halogens, have seven
valence electrons.
A pictorial representation of the number of valence electrons
on an atom can be shown with a Lewis
structure. The Lewis structure representation is very, very
important tool to enhance our view of simple chemical compounds.
Interestingly enough, our study of the reactivity of the
elements will begin with that group of elements that are the
least reactive–the inert gases. The secret to their
stability can be found in their electron configuration. As they
contain fully filled electronic shells/sublevels. Helium has a 1s2
configuration, Ne is 1s22s22p6
each having completly filled shells. In argon it is true the 3d
orbitals are unfilled, but those orbitals that are occupied are
filled to saturation. The electrons are held tightly and as we
have noted from ionization energies and electron affinities there
is little desire to add to or remove electrons from their
arrangements. Quite clearly these configurations exhibit unusual
stability.
However, this stability of the elemental form does not occur
in the two groups which flank the noble gases–the halogens
and the alkali metals. We've already seen demonstrations showing
the reactivity of members of both of these groups of elements.
Moreover, although we have not studied these reactions, these two
groups have a particular propensity to react with each other. And
when doing so it is always to form a compound inwhich the atomic
ratio is 1:1. Sodium chloride, NaCl, is of course the most
familiar example.
All of the alkali metal halides share a number of common
distinguishing properties. They are all crystalline solids
with high melting points (600 – 1000 C). Suggesting they are
held together by strong forces. When the solids are melted the
liquid is found to conduct electricity. It is possible for
electrical current to be carried by charged particles. And it was
Faraday who carefully studied such behavior. Additionally he
found that when a current was passed through a liquid, in the
case of NaCl, sodium was attracted to the cathode (negatively
charged electrode) and the chloride was attracted to the anode
(positively charged electrode). All of this suggesting that the
sodium in sodium chloride was not ordinary but existed as charged
atoms, or ions, and they were called cations as they were
attracted to the cathode. Similarily the behavior of the chloride
suggested it was negatively charged and they were called anions.
These observations were supported by other experiments. In
general the alkali metal halides dissolve readily in water. The
solvent, H2O, promoting the collapse of the
crystalline solid and the separation of the charged ions. The
resulting solution which contains Na+ and Cl-
ions readily conducts electricity. Such soluble salts, which
conduct electricity, are called electrolytes. The
formation of the ions in solution by electrolytes is
characterized by the following equation;
NaCl(s) ---H2O --> Na+(aq)
+ Cl-(aq)
For sodium chloride there are ions in the pure liquid and in
solution, the ions must also be present in the solid. And we can
rely on more than inference to support our predictions. Solid
state characterization have revealed a characteristic arrangement
of charged ions whose structures is;
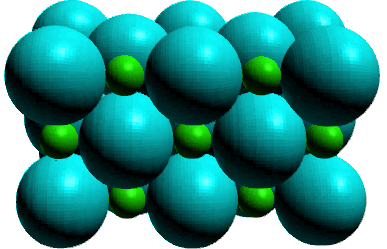
Because of the ion nature of these compounds they are called ionic
compounds. The interaction between the cation and the anion
that occurs in the solid state is called an electrostatic
attraction between the positively charged cation and
negatively charged anion. This electrostatic attraction is called
an ionic bond. How do we visualize the formation of the
charged ions? If we consider the reaction between a sodium atom
and a chlorine atom, we can write
Na + Cl -----> Na+ + Cl-
To understand the nature of the ions formed and the basis for
the ionic bond we can write the respective electron
configurations.
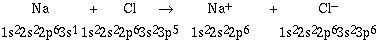
Another way to write the equation is to use
electron-dot symbols or Lewis symbols. These symbols are a
convenient way to indicate the number of valence electrons and
help keep track of electrons during bond formation. In the
equation we are considering sodium has 1 valence electron (1s22s22p63s1)
because of the single electron in its outer most shell. We can
write a Lewis structure for sodium as;
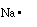
This indicates sodium has one electron in its
outer most shell. Chlorine has seven valence electrons in its
outer most shell (1s22s22p63s23p5).
We can write a Lewis structure for chlorine as;
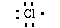
Sodium loses an electron and chlorine gains an electron. As a
result of the transfer of the electron each now has a noble gas
configuration and has little tendency to react further. The
sodium is so intent on achieving a noble gas configuration that
it will readily lose an electron. Chlorine is interested in
gaining an electron to achieve the noble gas configuration.
Recall that chlorine has a high tendency (electron affinity) to
gain an electron and sodium has a low ionization potential.
The new Lewis symbols are;

Also notice the new electron configuration for
the ions. Sodium is 1s22s22p6
and chlorine is 1s22s22p63s23p6.
Each has eight electrons in its outer most shell. Each has an
octet. The two elements have lost (sodium) or gained (chlorine)
electrons to achieve an octet of electrons. We will observe the
in simple terms much of the chemistry of the elements can be
understood in terms of this relatively simple magic number of
electrons. Of course hydrogen does not gain or lose electrons in
the same manner. Since its nearest noble gas is helium, hydrogen
will either lose (most often) one electron or gain one electron.
This is called the duet rule.
Remember the octet rule is simply a summary
statement of the observation that electrons are transferred such
that elements achieve an octet of electrons. It is not a driving
force in itself. It is simply a rule to help us make predictions
and to summarize a large amount of data with a simple statement.
Another example can be given to examplify this
behavior. We'll consider the reaction between a magnesium atom
and an oxygen atom.
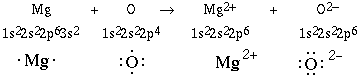
These two ions are both isoelectronic (same electronic
configuration) with the neon atom. Again the ratio of ions in
magnesium oxide will be 1:1 because of the identical magnitude of
charge on both atoms. This is an example inwhich magnesium is oxidized
(loss of electrons) and oxygen is being reduced (gain of
electrons).
Notice the electron lost by the metal is removed from the
outer most orbital, the 3s orbital. For transition elements the
loss of the electron is also from the outer most orbital, but
care must be taken. Consider the element manganese, Mn, with the
electron configuration of 1s22s22p63s23p64s23d5.
In the transition metal elements it is unlikely, and the
chemistry of these elements bare this out, that electron loss
will occur to attain an inert gas configuration. However, the
transition elements do lose electrons. When this occurs the first
electron to be lost comes from the 4s orbital, as does the second
electron, then any further electron loss comes from the d
orbitals. So Mn2+ has the electron configuration of 1s22s22p63s23p63d5,
Mn3+ has the electron configuration of 1s22s22p63s23p63d4.
Lattice Energy: Strengths of Ionic Bonds
Although there are several important factors which must be
considered in determining the energy associated with forming
ionic bonds, the lattice energy is were we will focus. The
lattice energy is defined as the energy liberated in the
following reaction;
Mn+(g) + Xm-(g)
---> MmXn(s)
Lattice Energies of Alkali Metal Halides
(kJ mol-1)
| |
F-
|
Cl-
|
Br-
|
I-
|
Li+
|
1036
|
853
|
807
|
757
|
Na+
|
923
|
787
|
747
|
704
|
K+
|
821
|
715
|
682
|
649
|
Cs+
|
785
|
689
|
660
|
630
|
Rb+
|
740
|
659
|
631
|
604
|
Lattice Energies of Salts of F-
(kJ mol-1)
| |
F-
|
Na+
|
923
|
Mg2+
|
2957
|
Al3+
|
5492
|
The lattice energy is the energy liberated when oppositely
charged ions in the gas phase come together to form a solid. So
for sodium chloride the lattice energy is 787 kJ mol–1.
This is the energy liberated when Na+ and Cl–
ions in the gas phase come together to form the lattice of
alternating Na+ and Cl– ions in a NaCl
crystal.
The charges and sizes of the ions are the critical factors
which determine the magnitude of the lattice energy. The lattice
energy is related to the potential energy of two interacting
charges which is given in the mathematical equation;
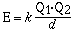
Q1 and Q2 are the magnitudes of the
charge on the particles in coulombs and d is the distance between
centers in meters. The constant k has a value of 8.99 x 109J
m C–2. From this relationship the magnitude of
the lattice energy is directly related to the charge on the ions
and inversely related to the ionic radii of the ions. The
combination of a small anion and a small cation liberates more
energy compared to the combination of large ions. In general
holding one of the ions constant the trend in lattice energy is
reflected in the change in size of the
counterion. The strength of the ionic bond also depends on
the magnitude of the charge on the cation or anion. In the case
of the fluoride compounds of sodium, magnesium and aluminum there
is a dramatic difference in the lattice
energy with cation charge.
It is the formation of the ionic bond which is the driving
force for this reaction. In the solid sodium chloride
considerable energy is liberated as a result of the arrangement
of the sodium and chloride ions.
To generalize this electron lose and electron gain behavior
we should note that Group IA metals, with their single valence
electron will easily lose the electron to form a +1 cation. A
behavior that continues down the Group. Group IIA metals will
lose their two valence electrons to form M2+ cations.
When this occurs the cations are isoelectronic with the
preceeding noble gas. Group IIIA metals form M3+
species, although as we go down the Group we do see M+
species appearing. Removing 3 electrons requires considerably
more energy, and as a result we see fewer ionic compounds in
Group IIIA compared to Group IA and Group IIA.
Group IVA and VA exhibit little tendency to lose or gain
electrons to form ionic bonds. Members of these two groups prefer
to share electrons.
In Groups VIA and VIIA, elements with high electron
affinities, we see gain of electrons to become isoelectronic with
the next noble gas. Group VIA gaining two electrons and Group
VIIA gaining one electron.
Ionic Radii
Before continuing we should consider the radii of the ions (common
cations/common anions) formed when electrons are lost or
gained by neutral atoms. The cation formed when one or more electrons are lost
has a smaller ionic radius compared to the neutral atom. The loss of electrons
increases the effective nuclear charge of the electrons remaining on the ion
and they experience a greater attraction towards the nucleus. Adding of one
or more electrons to a neutral atom, to form an anion, decreases the effective
nuclear charge of the valence electrons and the ionic radius is greater compared
to the neutral atomic radius. For isoelectronic ions the greater the positive
charge the smaller the ionic radius. While for the isoelectronic ions the greater
the negative charge the larger the atomic radius.
Nuclear Charge
|
Element
|
Electronic Configuration
|
Effective Nuclear Charge
|
Valence Electrons
|
Core Electrons
|
4+
|
Be
|
1s22s2
|
2+
|
2
|
2
|
5+
|
B
|
1s22s22p1
|
3+
|
3
|
2
|
5+
|
B+
|
1s22s2
|
3+
|
2
|
2
|
10+
|
Ne
|
1s22s22p6
|
8+
|
8
|
2
|
9+
|
F
|
1s22s22p5
|
7+
|
7
|
2
|
9+
|
F–
|
1s22s22p6
|
7+
|
8
|
2
|
Same number of valence electrons in Be and B+,
however the valence electrons in B+ 'see' a greater
effective nuclear charge and are attracted closer to the
nucleus––smaller radius than Be.
Same number of valence electrons in Ne and F–,
however the valence electrons in F– 'see' a
smaller effective nuclear charge and are not held as tightly by
the nucleus––larger radius than Ne.
Some elements which form ionic compounds are too far removed
from a noble gas to readily achieve the rare gas configuration.
Members of the transition metals series would have to lose a
large number of electrons to become isoelectronic with the
nearest noble gas. Most transition metals form cations of 2+ and
3+. This suggests that stable ions can exist which do not
duplicate the electronic arrangements of the noble gases. Some of
the ions can be characterized by filled subshells, for example,
Zn2+ [Ar]3d10, Ag+ [Kr]3d10
and Pb2+ [Xe]6s25d10. It is
therefore more difficult to predict most stable ions in the
transition metals. Instead their chemistry is a collection of a
large variety of ionic compounds.