Bohr was suggesting the energy of the electron was quantized,
that is the electron could only exist in certain allowed energy
levels.
In order to reproduce the same equations determined empirical
by Rydberg and the others, Bohr formulated a model of the hydrogen atom where the
electron could only occupy certain energy states called
stationary states.
Bohr's mathematical relationship which expressed these ideas
is;
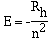
Note Rh is = 2.18 x 10-18 J. The
negative arises because Bohr assumed that when the electron is
removed from the atom the system would be defined as having zero
energy. But when the electron is around the nucleus, the system
is more stable and therefore, must be at a lower energy.
Therefore, the negative sign. 'n' in the equation is a
special number called a quantum number. It can only have integer
values from 1 to infinity. The quantum number is also a way of
labeling each orbit available to the electron. The first orbit is
referred to as the ground state, or the state of the atom at its
lowest energy.
The equation suggested by Bohr can generate the equation
obtained by Rydberg and the others by recalling,
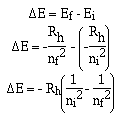
Bohr was also able to calculate the radius of the electron in
each of the allowed orbits. These are given by the equation,
r = n2 (5.30 x 10-11 m)
If the energy values of the first 10 orbits are calculated
one can see the energy gets closer and closer to zero, and the
energy of the orbits as n get larger get closer together.
This can best be seen when the energies are plotted on a graph.
Simultaneously the radius of the orbit of the electron gets
larger and larger. So as the electron gets further away from the
nucleus the energy of the electron inthe orbit gets closer to
zero.
So if we plot the energy levels for the electron in the
hydrogen atom we have a series of levels that look like;
n
|
Energy (Joules)
|
Radius(nm)
|
....
|
0 J
|
|
6
|
-0.61 x 10-19
|
1.90
|
5
|
-0.87 x 10-19
|
1.32
|
4
|
-1.36 x 10-19
|
0.846
|
3
|
-2.42 x 10-19
|
0.476
|
2
|
-5.45 x 10-19
|
0.212
|
6
|
-2.18 x 10-18
|
0.0529
|
Note as n increases the difference in energy between
subsequent levels gets smaller while the separation between
energy levels gets larger. The radius can be though of as the
distance of the electron, in the particular energy level, from
the nucleus. The electron can occupy any of these energy levels,
which are further and further from the nucleus. Bohr's model can
now be used to explain the observed emission spectrum for
hydrogen. To do this Bohr postulated that the electron could
change energy only by going from one energy level to the next.
Such a change was called a transition.
The absorption and the emission spectrum of hydrogen could
now be explained in the following way. If the electron is in the
ground state of the atom and a photon of light of the exact
correct frequency (energy) is incident on the atom the electron
can absorb the energy of the photon and is excited to a higher
energy level, or higher orbit. Later the electron can fall from
the orbit of high energy back to the ground state by re-emitting
the photon of light of the same frequency as the original photon
which excited the electron. For example, if an electron were in
the n = 2 energy level and fell to the n = 1 energy
level the difference in energy is;
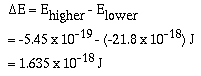
Recall that,
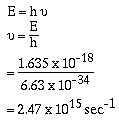
The same value for the first line in the Lyman series of the
emission spectrum of the hydrogen atom. The photon that is
released in this transition from the n = 2 level to the
n = 1 level has a frequency of 2.47 x 1015 sec-1.
So the electron falling from any energy level to the n = 1
level reproduces the Lyman series of lines in the emission
spectrum of the hydrogen atom. An electron falling from any level
to the n = 2 level, reproduces the Balmer series, etc.
Remember for the electron to go from a lower energy level to a
higher energy level an absorption of energy must occur. But to
make the particular transition it must absorb a photon of the
exact energy. No more, no less.
When an electron moves from one energy level (stationary
state) to another it either absorbs energy or emits energy. When
it is in any of the energy levels it does not spontaneously emit
energy and spiral into the nucleus.
As accurate as Bohr's model of the atom was in reproducing
the discrete lines in the emission spectrum of the hydrogen atom,
there was still a problem. As spectroscopic techniques were
refined and developed, faint new lines began to appear in the
emission spectrum that began to severely tax Bohr's model. In
addition, it was shown that Bohr's model we unsuccessful in
explaining the emission spectrum of the next element in the
periodic table-helium. Bohr's model of the atom was simple, but
it is incomplete. What is required is a new and different way to
thing of the atom to better understand the messages it is sending
us.
Bohr's model worked for a simple system, but did not
completely explain additional detail associated with the emission
spectrum. To a point it worked. In these circumstances
modifications of the model are typical needed. The primary
modification of the Bohr model which resulted in our current view
of the electronic structure of the atom was to recognize the
electron exhibited wave properties as well as particle
properties. This rather simple statement was the result of
considerable labor. Let's go back to the origin of the ideas
which subsequently lead to this important modification of
describing the behavior of an electron in an atom.
In his study of the Photoelectric effect Einstein recognized
that light exhibited particle behavior. When light of specific
frequency was incident on a metal surface electrons were ejected
from the metal. The incident light in causing electrons to be
ejected was behaving as a particle. Light, electromagnetic
radiation, could not only be thought of as having wave
properties, but also particle properties. A packet of light
(photon) had a frequency, but it also had an energy given by;
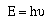
Now we began paying a price for our search to elucidate the
structure of the atom and a gentleman by the name of Louis Victor
de Broglie is responsible. What he did was to make the atom an
abstract and and difficult to imagine. He made the universe
probabilistic and indeterminate. Now understand that the universe
has not changed, but our view of it has.
The pivotal concept suggested by de Broglie, was that if
light could exhibit particle properties, and therefore, help us
explain phenomenon, then why could not matter have wave
properties. de Broglie was saying that matter, under certain
circumstances might also display appreciable wavelike properties.
He suggested that the wavelength of matter behaving in this
manner could be calculated using the relationship;
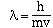
where 'm' is the mass of the particle and 'v' is its
velocity.
Called matter waves they should not be confused with the
waves of electromagnetic radiation. Matter waves are not radiated
into space or emitted by a particle; they are never dissociated
from the particle. The speed of matter waves is never the speed
of light, nor is it constant, but changes with the particle. de
Broglie suggested that electrons, because of their very small
mass and high velocity, could behave as waves. There existed
experimental evidence to support this idea. Even Thomson had
observed the beam of electrons in the cathode ray tube would cast
a shadow on the end of the tube when a piece of metal is placed
in the beam.
For years after de Broglie's postulate Clinton Davidson and
L. Germer investigating the scattering of electrons by atoms in a
crystal showed that the electron beam was reflected (diffracted)
in an identical way that X-rays were diffracted. Exhibiting the
wave-like character of the electron. Erwin Schrodinger, an
Austrian theoretical physicist, assumed that the wavelike
character of the electron could be incorporated into a model of
the atom. And here is where our world falls apart, and it becomes
very difficult to describe electron behavior in atoms. Because
now the medium has turned form pictures to probability and
quantum mechanics or wave mechanics. The language is differential
calculus, the words are symbols and numbers. The results are
complex, but nonetheless dramatic.
We will avoid that pit and try to describe the results. Try
is what I will do, because quantum mechanics changes the way we
think about the motion of particles as small as the electron.
Large objects moving slowly are easy to follow. Like a baseball.
A propeller turning slowly is also easy to follow, However, as we
speed up the blade it becomes difficult to know exactly where the
two ends of the propeller are located. Their movement describe a
region where, if we were to insert a pencil, we would expect to
encounter one end of the blade or the other. Outside its length
we would assume we would be safe. In the world of the atom the
motion of the electron is equally complex. It can be thought of
as smoothed out around the nucleus. We can not see an individual
electron or how fast it is going.
Werner Heisenberg showed by using quantum mechanics that it
is impossible to know both the position and velocity of the
electron;

Now this problem is only encountered in the realm of the
atom, we do not have such problem in the macroscopic world. (If
we did more of us would pay closer attention to the 'walk' and
'don't walk' sign at traffic signals). Heisenberg's uncertainty
principle says that because of the wavelike character of the very
small electron we can not know precisely where the electron is
when traveling along a path or trajectory.
If we accept that an electron has wave character than an
electron can be described as a wave. If we use a single wave to
decribe the velocity of the electron, as shown in the example, we
do not know where the electron is located exactly. We have an
idea of its vertical location, between the peak maxima, but we do
not know where it is located horizontally. If we use a number of
different waves and add them together we can better locate the
position of the electron, but we have no idea what the velocity
of the electron is because of the different velocities of the
waves that contribute to the electron. So Heisenberg's uncertain
principle concludes that we can not know both the position and
the velocity of an electron simultaneously. This means we can not
know exactly where the electron is an any instant.
We can not know how fast an electron moves in an atom (about
the nucleus). However, quantum mechanics does allow us to
describe the behavior of the atom in a statistical sense. We talk
about the probability of finding the electron at a certain point
in space in the vicinity of the atom. We can not describe the
electron as orbiting the nucleus. Because to do so suggests the
electron has a trajectory which suggests we can know both the
position and velocity of the electron. So in quantum mechanics we
describe the behavior of the electron in three-dimensional terms.
Instead of finding an electron in an orbit, as Bohr's model
suggested, we describe the electron in an orbital, which is a
3-dimensional orbit. But now the question becomes what
3-dimensional shape or shapes does an electron in a hydrogen atom
have?