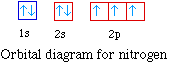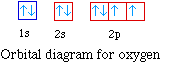A goal is to be able to identify the 'location' of every
electron in an atom as to its level, sublevel and orbital. This
can be accomplished by writing something we call an electron
configuration. Another way to identify the location of each
electron is via an orbital diagram. To designate the electron
configuration we use the level number and the letter of the
sublevel and a superscript number to represent the number of
electrons contained in the sublevel. Writing the electron
configuration requires that we recall how many orbitals are
contained in each type of sublevel
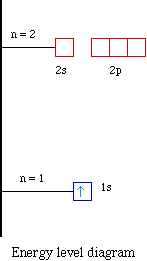
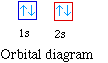
Forexample, hydrogen has one electron. Where is the electron
located? Our first assumption is the electron is located in the
level, sublevel and orbital with the lowest energy. We know the
orbital with the lowest energy is the 1s orbital. For example
hydrogen with one electron has an electron configuration of 1s1.
The orbital diagram for hydrogen can be represented in the
following way.
This notation uses a box to represent the orbital, the label
for the orbital and an arrow to represent the electron. The
electronic configuration for hydrogen can be written as 1s1.
This is a short-hand notation which identifies the level, the
sublevel and the number of electrons in the sublevel. We can also
display the energy level diagram for the hydrogen atom. A portion
of the energy level diagram is shown,
So we have three ways to represent the electron arrangement
in an atom. The orbital diagram, the electron configuration and
the energy diagram. All three ways are useful.
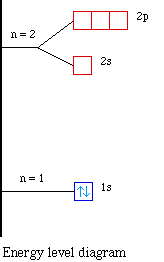
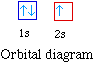
The next atom is helium with 2 electrons. So the second
electron could go into the 1s orbital with the opposite spin of
the first electron or it could go into the next orbital in the n
= 2 level. It turns out that the energy required to accommodate
two electrons in the 1s orbital is significantly less than the
energy required to place the second electron into the higher
energy n = 2 level. The orbital diagram for helium is,
So while hydrogen has the electron configuration of 1s1,
helium has the electron configuration of 1s2. The
energy diagram for helium is shown as here. Notice that there has
been a change in the relative energies of the 2s and 2p orbitals.
This is an important point that must be addressed at this point.
In the hydrogen atom the sublevels in each principle level
are degenerate. In multi-elecron atoms the degeneracy of the
energy of the sublevels is lost.
When the third electron is to be placed it must go into the
second level. The first level is filled and can not accommodate
any more electrons. When the electron is added to the second
level it can go into the 2s orbital or the 2p, the question is
which orbital is the electron placed? The primary criteria, the
Aufbau principle, states the electrons are to be placed into the
orbital of lowest energy. So we must consider which orbital, when
the electron is placed into it, has the lowest energy? This is
answered by considering some complicated mathematical
calculations. The essence of these calculations is that when an
electron is placed into the 2s orbital the electron is likely to
spend more time closer to the nucleus than an electron in a 2p
orbital. If the electron spends more time closer to the nucleus
the electron will experience a greater attraction to the nucleus
and it is lower in energy. It can be stated the 2s orbital
penetrates closer to the nucleus than does a 2p orbital.
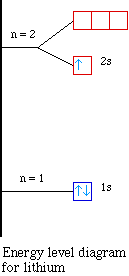
So the orbital diagram for lithium is shown below. The
electron configuration for lithium is 1s22s1.
The energy level diagram, on the left shows the relative
energy of the 2s and 2p orbitals based on the ability of the
sublevels to penetrate to the nucleus.
The next element is beryllium which has four electrons. The
orbital diagram for beryllium is shown here. The electron
configuration is 1s22s2. The fourth
electron is placed in the 2s orbital. The energy required to pair
the first 2s electron is less than the energy required to place
the electron into the 2p orbital.
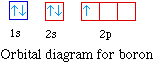
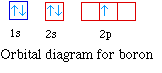
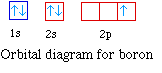
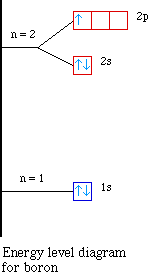
The next element is boron with 5 electrons. The orbital
diagram for boron as shown has the one electron in the 2p
orbital. The electron can be placed in any of the three 2p
orbitals. The electron configuration for boron is 1s22s22p1.
The energy level diagram for boron is show below.
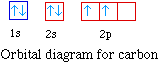
For the next element, carbon, the sixth electron must be
placed in the correct orbital. The question becomes whether the
next electron should be pair the other electron or whether the
electron should be placed in an empty 2p orbital.
According to Hund's rule the most stable arrangement in a set
of degenerate orbitals is that with the most number of unpaired
electrons. So carbon has two unpaired electrons.
The electron configuration for carbon is 1s22s22p2.
Notice the electron configuration does not clearly indicate the
number of unpaired electrons in the element. The number of
unpaired electrons is evident from the orbital diagram. The
orbital energy diagram for carbon is shown below.
Nitrogen has seven electrons. The placement of the next
electron must follow Hund's rule. The orbital diagram shows three
unpaired electrons. The electron configuration for nitrogen is 1s22s22p3.
For oxygen the eighth electron must pair with one of the
electrons in the 2p orbitals. The orbital diagram for oxygen is
shown on the left. The electron configuration for oxygen is 1s22s22p4.
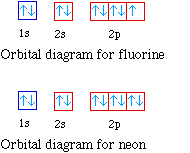
The orbital diagrams for fluorine and neon are shown. The
next two electrons continue to pair those electrons that are
unpaired to fill up the 2p orbitals.
With neon the second level is filled with electrons.
Completed levels are a characteristic of all noble gases. If we
look at the energy level diagram for neon the completed second
level means the next electron must go into the third level. In
the hydrogen atom the three sublevels, 3s, 3p and 3d were all
degenerate in energy. In the multi-electron atom the three
sublevels do not have the same energy. The relative energies of
the three sublevels again depend on the ability of the electron
to penetrate to the nucleus. As in the case of the second level
the 3s orbital is lower in energy than the 3p which is lower in
energy compared to the 3d.
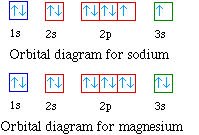
So as we progress from sodium across the period to argon the
electrons are placed in the orbitals just as they were for the
second period.
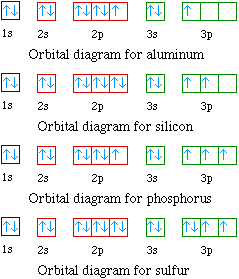
The orbital diagrams for the eight elements are shown below.
The electron configurations for the next eight elements are
as follows:
Na 1s22s22p63s1
Mg 1s22s22p63s2
Al 1s22s22p63s23p1
Si 1s22s22p63s23p2
P 1s22s22p63s23p3
S 1s22s22p63s23p4
Cl 1s22s22p63s23p5
Ar 1s22s22p63s23p6
When we come to potassium more interesting changes are
observed. Chemically potassium behaves like sodium, as an alkali
metal. It appears the next electron is in an s orbital, not a 'd'
orbital. It turns out the energy of the 4s orbital is very close
to the energy of the 3d orbital at potassium. But the energy of
the 4s orbital is lower in energy compared to the 3d. So the next
electron is placed into the 4s orbital. At calcium the electron
is paired. For scandium we might consider whether the electron
goes into the 3d or the 4p. It turns out the energy of the 3d is
lower than the 4p so the d sublevel begins to fill with scandium.
The electron configuration for scandium is [Ar]4s23d1.
As electrons are added in titanium and vanadium the configuration
is [Ar]4s23d2 and [Ar]4s23d3.
The next element, chromium, would be expected to have a
configuration of [Ar]4s23d4, however this
is not the case. It turns out that as a result of the similarity
in energy of the 4s sublevel and the 3d sublevel in this group
that an interesting phenomena occurs at chromium. Instead of
[Ar]4s23d4 the electron configuration is
[Ar]4s13d5. We might suggest that a
half-filled d sublevel has extra stability. The next element,
manganese, the additional electron is added to complete the
half-filled 4s sublevel and the configuration is [Ar]4s23d5.
From iron through nickel ([Ar]4s23d6,
[Ar]4s23d7, [Ar]4s23d8)
the electrons spin-pair in the 3d sublevel. At copper another
reversal occurs. The electron configuration for copper is [Ar]4s13d10
not [Ar]4s23d9. Zinc has an electron
configuration of [Ar]4s23d10.
At gallium we begin filling the 4p sublevel and continue to
krypton. Rubidium fills the 5s, yttrium the 4d and indium the 5p.
Cesium fills the 6s and lanthanum bigins the first available f
sublevel, the 4f. The f sublevel is filled from lanthanum through
ytterbium. Throughout this period there are strange reversals of
configurations. The details of these changes are not critically
important to us.
Having gone through this exercise it is interesting to study
the periodic table in light of the position of the valance
electrons of each atom. The periodic table displayed uses color
to denote the location of the outer-most electrons. All of the
alkali metals have the outer most, or valence electron, in an s
orbital. For lithium the outer most electron is in the 2s
orbital, for sodium the 3s, for potassium the 4s, etc. We say the
general electron configuration for the alkali metals is ns1.
For the alkaline earth elements it is ns2.
The periodic table can be used to write the electron
configuration for any element. The trick is locate the particular
element in the correct level and sublevel. The level numbers are
located to the left of each period. The sublevels are identified
by noting the section of the periodic table the element is
located. (Describe the colors of the periodic table and do an
example using the periodic table.)
It is useful to understand the observed trends in particular
physical properties of the elements in relation to their location
in the periodic table. The idea is note the physical properties
and then understand the observe behavior in terms of the nature
of the electron.