A common laboratory preparation of O2 involved the decomposition
of hydrogen peroxide, H2O2, according to the equation,
2H2O2(aq) ---> 2H2O(l)
+ O2(g)
If 240 mL of O2 at 23 ūC and at 0.965 atm pressure are collected
over a sample of water at the same temperature determine the number of moles
of O2 obtained in the reaction.
Solution:
Since the oxygen is collected by displacement of water as the oxygen is collected
water is displaced from a text tube initially filled with water.
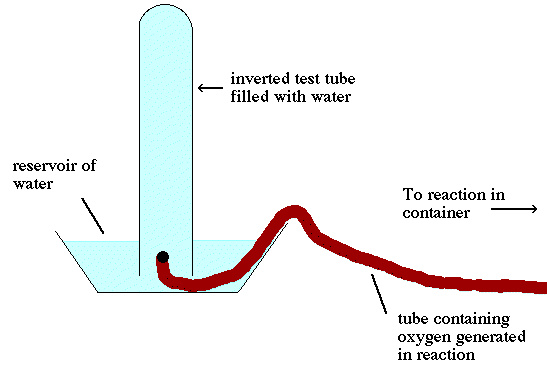
(see Figure 5.12 on page 196 in your textbook). So as the oxygen gas fills
the test tube water escapes in to the vapor phase and mixes with the oxygen
in the gas phase.

A Dalton's Law problem!

So the total pressure exerted by the gas above the water in the test tube
is the sum of the pressure due to the water in the vapor phase and the oxygen
in the vapor phase.
PT = PO2 + PH2O
At 23 ūC the PH2O is 21.0 mmHg this can be obtained from a table
of vapor pressures.
Sine the question asks for the number of mol of oxygen we must eliminate the
water in the gas phase above the sample,
PO2 = PT - PH2O = 733 mmHg - 21.0 mmHg = 712 mmHg
(.937 atm)
Now that we have the pressure of only oxygen in the test tube we can calculate
the mol of oxygen;
nO2 = PV·(RT)-1 = (0.937 atm .
0.240 L)· (0.0821 L·atm mol-1·K-1
· 296 K)-1 = 9.25 x 10-3 mol O2