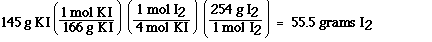According to the chemical equation
2CuCl2(s) + 4KI(s) ---> 2CuCl(s)
+ 4KCl(s) + I2(s)
a) how many grams of iodine are produced when 145 g of KI
react with excess copper(II) chloride?
Answer
This problem is slightly different compared to the earlier
problems. The most important change is the amount of starting
material is given in grams and not moles. To solve this problem
we must first convert the mass of potassium iodide to moles using
the molar mass of potassium iodide. Then we can use the
stoichiometric factor to convert from moles of potassium iodide
to moles of iodine. This stoichiometric ratio is obtained from
the balanced chemical equation. According to the balanced
chemical equation four mol of potassium iodide react to form 1
mol of iodine. Potassium iodide and iodine always combine in this
ratio according to this equation. After determining the moles of
iodine we can convert moles to grams of iodine using the molar
mass of iodine.