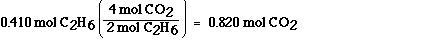According to the chemical equation
2C2H6(g) + 7O2(g) --->
4CO2(s) + 6H2O(g)
b) how many mol of carbon dioxide will form by the complete
combustion of 0.410 mol of C2H6?
Answer
The approach is identical to that used in part a). We must
use the stoichiometric ratio between carbon dioxide and ethane to
answer the question. This ratio is obtained from the balanced
chemical equation. According to the balanced chemical equation
four mol of carbon dioxide are formed when two mol of ethane, C2H6,
react. Carbon dioxide and ethane always combine in this ratio
according to this equation. We can use this relationship as a
unit conversion factor to convert between mol of ethane and mol
of carbon dioxide.