According to the chemical equation
4KO2(s) + 2CO2(g) ---> 2K2CO3(s)
+ 3O2(g)
d) how many formula units of potassium superoxide must react
to form 27 molecules of O2 assuming excess carbon
dioxide?
Answer
According to the balanced chemical equation when three
molecules of dioxygen are formed when 4 formula units of
potassium superoxide react (assuming excess carbon dioxide).
Dioxygen and potassium superoxide always combine in this ratio
according to this equation. We can use this relationship as a
unit conversion factor to convert between potassium superoxide
and dioxygen.
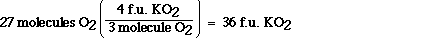
This conversion between dioxygen and potassium superoxide
tells us the thirty six formula units of potassium superoxide are
formed when twenty seven molecules of dioxygen react.