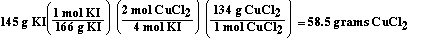According to the chemical equation
2CuCl2(s) + 4KI(s) ---> 2CuCl(s)
+ 4KCl(s) + I2(s)
b) how many grams of copper(II) chloride are required to
react with 145 g of KI?
Answer
This problem is similar to the first problem in this group.
Once again the amount of starting material is given in grams and
not moles. To solve this problem we must first convert the mass
of potassium iodide to moles using the molar mass of potassium
iodide. Then we can use the stoichiometric factor to convert from
moles of potassium iodide to moles of copper(II) chloride. This
stoichiometric ratio is obtained from the balanced chemical
equation. According to the balanced chemical equation four mol of
potassium iodide react to form 2 mol of copper(II) chloride.
Potassium iodide and copper(II) chloride always combine in this
ratio according to this equation. After determining the moles of
copper(II) chloride we can convert moles to grams of copper(II)
chloride using the molar mass of copper(II) chloride.