# of bonding groups/domains on 'central' atom |
# of lone pair electrons on 'central' atom |
|
|
|
2 |
0 |
linear |
linear |
180 |
3 |
0 |
trigonal planar |
trigonal planar |
120 |
2 |
1 |
trigonal planar |
bent |
less than 120 |
4 |
0 |
tetrahedral |
tetrahedral |
109.5 |
3 |
1 |
tetrahedral |
trigonal pyramidal |
less than 109.5 |
2 |
2 |
tetrahedral |
bent |
less than 109.5 |
5 |
0 |
trigonal bipyramidal |
trigonal bipyramidal |
90, 120 and 180 |
4 |
1 |
trigonal bipyramidal |
seesaw |
90, 120 and 180 |
3 |
2 |
trigonal bipyramidal |
90 and 180 |
|
2 |
3 |
trigonal bipyramidal |
180 |
|
6 |
0 |
octahedral |
90 and 180 |
|
5 |
1 |
octahedral |
90 and 180 |
|
4 |
2 |
octahedral |
90 and 180 |
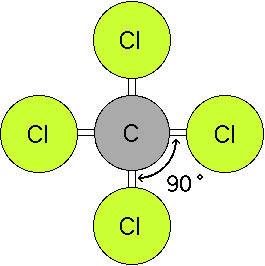
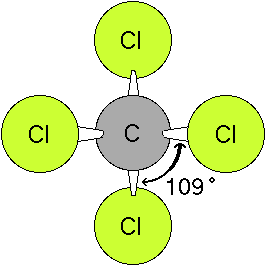
Here are some examples of the 3-dimensional structure in simple compounds. (Note: you will need the MDL ChemScape Chime Plugin to view these files.)
Here are some examples of the 3-dimensional structure for more complex compounds. (Note: you will need the MDL ChemScape Chime Plugin to view these files.)
Here is the Purdue site we used in class.
Here are some VSEPR animations that do not require a plug-in to view.