|
Chapter 11: Introduction to Modern Atomic Theory
The next element is lithium. Lithium has three
electrons.
The first two of these three electrons are placed
into the 1s orbital.
Where do we place the third electron?
The next lowest energy level is the second level. But
there are two sublevels in the second level. Before
placing the third electron into the second level the two
sublevels are degenerate in energy (have the same
energy).
When we place the third electron into the second
level the energy of the two sublevels change relative to
each other. The 2s sublevel drops in energy and the 2p
sublevel increases in energy. So the third electron goes
into the orbital in 2s sublevel.
Lithium has an electron configuration of 1s22s1.
The orbital diagram looks like,
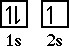
In the orbital diagram we can place each orbital on
the same line.
Would like to practice
writing an electron configuration?
*NOTE: This file will load very slowly if you are
trying to download on a 56K modem. View this movie using
QuickTime 4 on the campus network.
|