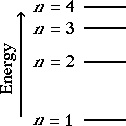|
Chapter 11: Introduction to Modern Atomic Theory
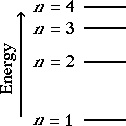 |
An energy level diagram for the Bohr
model of the hydrogen atom is shown on the left.
The ground state, lowest energy level, is n
= 1. As n increases the energy of the
level gets higher. As n increases the
energy levels get closer together.
|
In the Quantum Mechanical model of the hydrogen atom
we view the electron as having wave properties. This
introduces some complexity to the energy level diagram.
Closer study of the emission spectrum of the hydrogen
atom also reveals fine-lines suggesting the energy level
diagram for the hydrogen atom is more complex.
For example,
the first level ( n = 1) has one sublevel,
the second level contains two sublevels,
the third level contains three sublevels,
the fourth level contains four sublevel, etc.
It also turns out that each of these sublevels
contain orbitals (the three dimensional region of space
where the probability of finding the electron is high).
*NOTE: This file will load very slowly if you are
trying to download on a 56K modem. View this movie using
QuickTime 4 on the campus network.
|