![]()
![]()
Particle |
Charge |
Mass |
electron |
-1.6022 x 10-19 Coulombs |
9.109 x 10-31 kilograms |
proton |
-1.6022 x 10-19 Coulombs |
1.673 x 10-27 kilograms |
neutron |
0 |
1.675 x 10-27 kilograms |
![]()
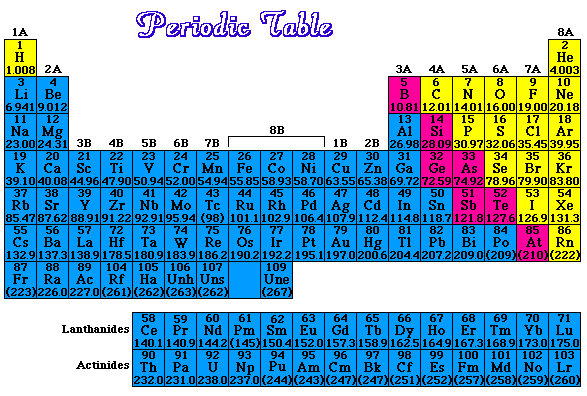
How many protons and how many electrons in each of the following elements?a) Cb) Fec) Hgd) UAnswers |
![]()
Symbol |
Atomic Mass (u) |
% Abundance |
1H |
1.0078250 |
99.985% |
2H |
2.0141017 |
0.015% |
The weighted average atomic mass of hydrogen is;weighted average = (atomic mass fractional abundance)1 + (atomic mass fractional abundance)2substituting,weighted average = (1.0078250 0.99985)1 + (2.0141017 0.00015)2 = 1.00797 u |
Determine the relative weighted average atomic mass of the element neon, given the following information,
Answer |
Complete the following table;
Answers |
![]()
Given that metals lose electrons, write the symbol for the most common cation for each of the following.a) Csb) Fec) BaAnswers |
![]()
Indicate whether each of the following compounds is ionic or covalent.a) KFb) CO2c) C6H12O6d) Fe(NO3)2e) SnCl2AnswersEach of the following ionic compounds dissolve in water. Write the formula for the cation and anion in each compound. (NOTE: be sure to include the charge on each ion.)a) BaCl2b) KNO3c) NH4ClO4d) NaC2H3O2Answers |