The first property to explore is atomic radius. The immediate
question is what is an atomic radius. Our quantum mechanical
description of an atom suggests a very broad region for finding
the electron. It is difficult to define a sharp boundary for
distance between the electrons in any particular atom and the
nucleus. So some approximations are made in determining this
parameter. For example, the distance between the two chlorine
atoms in Cl2 is known to be 1.988 Å. To get the
atomic radius we assume the distance between the two nuclei is
the sum of two chlorine atomic radii. Therefore the atomic radius
of chlorine is 0.994 Å. Additional distances can be obtained
from other distances between atoms. Care must be taken in these
types of determinations however.
After collecting large amounts of data generally accepted
atomic radii are known for most elements in the periodic table.
Atomic radii determine this way are also called covalent radii.
(we'll discuss the term covalent in the next chapter) Atomic
radii for metals can be estimated from the distance between metal
atoms in the pure solids. Figure 11.32 in your text graphically
summarizes the observed atomic radii for most elements.
Looking at the table notice from lithium to cesium, going
down the group, the atomic radius increases. This is as expected
as the valence electron is located further and further from the
nucleus. Each of the elements in a group have the same number of
valence electrons. In the case of Group I there is one valence
electron. In lithium this single valence electron is found in the
second level, in sodium the single valence electron is located in
the third level. The third level is higher in energy AND further
away from the nucleus. Therefore a sodium atom is larger than a
lithium atom. The argument is the same for other higher members
of the group.
Also notice that going across a period the atomic radius
decreases. We can understand this trend by considering that as we
go across a period each electron added is going into the same
level. For example look at the electron configuration for the
eight elements in the third period.
Na 1s22s22p63s1
Mg 1s22s22p63s2
Al 1s22s22p63s23p1
Si 1s22s22p63s23p2
P 1s22s22p63s23p3
S 1s22s22p63s23p4
Cl 1s22s22p63s23p5
Ar 1s22s22p63s23p6
Sodium has one valence electron and as we move across to
argon with eight valence electrons. Remember, and this is
important, the valence electrons are in the outer most level of
the atom (the furthest from the nucleus). Remember also that as
we move from sodium to argon, the nuclear charge, the number of
protons in the nucleus, is also increasing. Sodium has 11 protons
and argon has 18 protons. Each time we add a proton to a the
nucleus the electron (which are negatively charged) feel a
greater attraction to the nucleus. Since valence electrons are
all in the same level, they feel a greater attraction to the
nucleus as we move across the period. If the valencce electrons
feel a greater attraction they are pulled in closer to the
nucleus and the atom gets smaller. So the one valence electron in
sodium feels a certain attraction to the nucleus. Magnesium has
two valence electrons, both in the same level, and both of these
electrons feel a greater attraction to the nucleus because the
nuclear charge has increased from going from sodium to magnesium.
You might ask why doesn't the radius get smaller as we go
from sodium to potassium? Potassium has a greater nuclear charge
compared to sodium. You're right potassium does have a greater
nuclear charge compared to sodium. The difference is the valence
electron in potassium is in a different and HIGHER level compared
to the valence electron in sodium. So the valence electron in
potassium is further away and can not feel the higher nuclear
charge. When going across a period each added electron goes into
the SAME level. SInce the nuclear charge is also increasing the
valence electron feel a greater attractio to the nucleus and the
atom's size gets smaller.
Another important trend which is related to the periodic
table is the ionization energy for an element. It is related to
the energy required to remove an electron from an atom. Clearly a
multi-electron atom would have many ionization energies. So by
definition the first ionization energy is defined as the energy
required to remove the outer most electron from a neutral atom in
the gas phase. An equation can be written to show this
definition,
M(g) -> M+(g) + 1e-
The second ionization energy is the energy required to remove
the next outer electron from the singly charged ion.
M+(g) -> M2+(g) + 1e-
Subsequent removal of electrons can also be written and
determined.
As you would expect each successive removal of an electron
requires more energy. As electrons are removed the remaining
electrons experience a greater attraction to the nucleus. As
shown in the table the ionization energies increase with each
successive removal. Notice that there occur major changes as
successive electrons are removed. For magnesium there is a large
change between the second and third electrons, for aluminum
between the third and fourth, for silicon between the fourth and
fifth. Magnesium has the electron configuration of 1s22s22p63s2.
The first two electrons are removed from the third level. The
third electron is removed from the second level. Electrons in
lower levels feel a greater attraction to the nucleus and are
more difficult to remove.
Looking at the table suggests the valence electrons, the
outer most electrons, require considerably less energy to remove.
This suggests that the outer electrons are much easier to remove
from the atom compared to the inner level electrons. The inner
level electrons are too tightly bound to be involved in chemical
bonding.
TABLE Successive values of ionization energies (I) for
the elements sodium through argon (kJ/mol)a
Element
|
I1
|
I2
|
I3
|
I4
|
I5
|
I6
|
I7
|
Na
|
490
|
4560
|
|
|
|
|
|
Mg
|
735
|
1445
|
7730
|
|
|
|
|
Al
|
580
|
1815
|
2740
|
11,600
|
|
|
|
Si
|
780
|
1575
|
3220
|
4350
|
16,100
|
|
|
P
|
1060
|
1890
|
2905
|
4950
|
6270
|
21,200
|
|
Now consider the variation in the first ionization potentials
and position of the element in the periodic table. If we look at
a plot of 1st ionization versus atomic number certain trends are
evident. The figure below shows the 1st ionization energies.
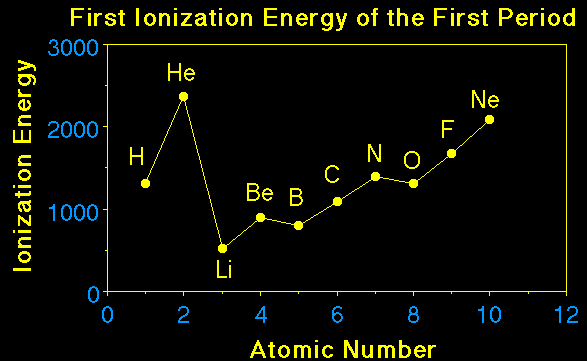
Notice the general trend is for the ionization energy to
increase going across a period. This makes sense if we recall the
atomic radius decreases going across the period. If the atom gets
smaller it means the electrons feel a greater attraction to the
nucleus and it is more difficult to remove the electron.
When we look more closely at the trend in ionization energies
we see two deviations. Boron's ionization energy is lower than
beryllium's and oxygen's is lower than nirogen's. What gives? In
the first case, lets look at the energy level diagram for boron
and beryllium.
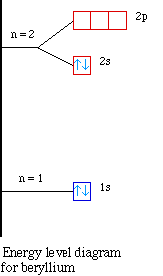
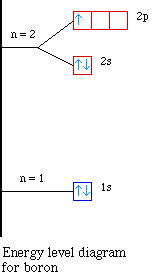
Notice the energy for the 2s and the 2p sublevels. The 2s
sublevel is lower in energy compared to the 2p sublevel. So when
we try to remove the first electron in boron it is easier because
it is higher in energy. Therefore the the first ionization energy
for boron drops when compared to beryllium. As we continue across
to carbon and then to nitrogen the electrons are going into the
same sublevel and we see the ionization energy increasing.
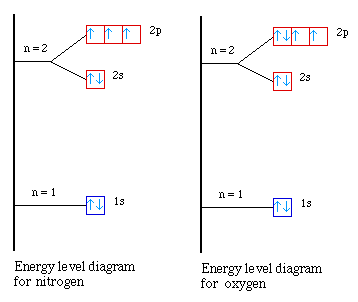
The second occurs from nitrogen to oxygen. What is going on
in this case? Again we need to look at the energy level diagrams
for the two atoms. In nitrogen all of the 2p orbitals are
half-filled. When we go next to oxygen the electron must be
placed in one of the half-filled orbitals. But we know that it
requires some extra energy to place two electrons into the same
orbital. Since it takes slightly more energy to pair the
electrons it takes less energy to remove the paired electron in
oxygen. So we see a small decrease in the first ionization energy
when going from nitrogen to oxygen.




